Cystic fibrosis (CF) is an autosomal recessive disorder caused by mutations in the CF transmembrane conductance regulator (CFTR) gene, and affects an estimated one in 3600 live births in the USA. CF lung disease, which causes the majority of CF morbidity and mortality(Reference Davis1, Reference Gao, Kim and Yankaskas2), is characterised by pulmonary recruitment of inflammatory cells, primarily neutrophils, which secrete cytokines, oxidants and other pro-inflammatory factors(Reference Rahman and MacNee3). Airway secretions in patients with CF are characterised by consistently elevated levels of IL-8(Reference Noah, Black and Cheng4), the major neutrophil chemoattractant(Reference Richman-Eisenstat, Jorens and Hebert5). This perpetuates neutrophil recruitment and leads to a state of chronic inflammation and tissue injury(Reference Davis1, Reference Chmiel, Merger and Konstan6), further aggravated during exacerbations of CF lung disease.
Respiratory epithelial cells have increasingly been recognised as key participants of inflammatory responses in the airways. IL-8 is secreted by epithelial cells in response to pro-inflammatory stimuli, such as TNF-α, neutrophil elastase(Reference Nakamura, Yoshimura and McElvaney7) or bacterial products (e.g. lipopolysaccharide (LPS))(Reference DiMango, Zar and Bryan8, Reference Greene, Carroll and Smith9). The CF respiratory epithelium may be intrinsically pro-inflammatory(Reference Tirouvanziam, de Bentzmann and Hubeau10). Modulation of lung inflammation is a recognised therapeutic target in CF(Reference Konstan and Davis11), yet current approaches have had limited success or undesirable adverse effects(Reference Dinwiddie12, Reference Lands and Dauletbaev13). In addition to pulmonary inflammation, malnutrition has an important prognostic role in CF, and its correction is a central therapeutic goal(Reference Davis1, Reference Konstan, Butler and Wohl14).
Whey proteins (WP), a by-product of the cheese-making industry, possess nutritional benefits as a source of protein of high biological value(Reference Krissansen15). Whey products and whey-derived peptides have demonstrated a number of anti-inflammatory effects(Reference Brody16, Reference Clare and Swaisgood17). These anti-inflammatory effects include decreased cytokine release in rodent models of ischaemia–reperfusion(Reference Yamaguchi and Uchida18) and exposure to LPS(Reference Beaulieu, Girard and Dupont19). In addition, individual whey constituents, such as lactoferrin(Reference Lee, Farmer and Hilty20, Reference Hayashida, Kaneko and Takeuchi21) or glycomacropeptide(Reference Otani, Horimoto and Monnai22), and peptides released from these by pepsin–pancreatin hydrolysis(Reference Bruck, Gibson and Graverholt23) exhibit anti-inflammatory effects, such as suppression of tissue neutrophilia(Reference Shimizu, Dairiki and Ogawa24) or inhibition of inflammatory cytokine release(Reference Kanwar and Kanwar25).
The beneficial effects of whey are further potentiated by hyperbaric pressurisation, which induces conformational changes in WP(Reference Hendrickx, Ludikhuyze and Van den Broeck26). These conformational changes expose peptide sequences normally embedded in the hydrophobic core, rendering them more accessible to enzymatic digestion and exposing concealed sulfhydryl groups(Reference Tanaka, Tsurui and Kobayashi27). Our group has demonstrated that pressurisation of WP improves their in vitro digestibility, promotes the release of novel peptides by gastrointestinal digestive enzymes and enhances anti-inflammatory effects(Reference Vilela, Lands and Chan28).
These in vitro findings were also confirmed in clinical studies. Thus, a 2-week supplementation with pressurised whey increased the levels of glutathione, a crucial low-molecular antioxidant, in peripheral blood mononuclear cells(Reference Zavorsky, Kubow and Grey29). Further, we have reported that a 1-month dietary supplementation with pressurised whey improved nutritional status and markers of systemic inflammation in patients with CF(Reference Lands, Iskandar and Beaudoin30).
The aims of the present study were to: (1) investigate the potential anti-inflammatory and antioxidant effects of pressurised and native WP (nWP) hydrolysates in CF and non-CF respiratory epithelial cells and (2) explore the mechanisms by which pressurised and native whey exert their beneficial anti-inflammatory effects. We hypothesised that peptides available through intestinal absorption of digested whey act on respiratory epithelial cells to decrease IL-8 responses to pro-inflammatory and pathogenic stimuli, and that this inhibition is enhanced by the pressurisation of whey.
Materials and methods
Reagents
Inpro 90 Whey Protein Isolate, purchased from Vitalus Nutrition, had the following composition: protein (dry basis) ≥ 93 %; β-lactoglobulin 43–48 %; glycomacropeptide 24–28 %; α-lactalbumin 14–18 %; bovine serum albumin 1–2 %; Ig 1–3 %; lactoferrin < 1 %. Pepsin from porcine stomach mucosa, porcine pancreatic trypsin, bovine pancreatic chymotrypsin, porcine intestinal peptidase, LPS and fluorescein isothiocyanate (FITC)-LPS from Escherichia coli (O55:B5), 5,5′-dithio-bis(2-nitrobenzoic acid) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were obtained from Sigma-Aldrich. Amicon ultrafiltration membranes (molecular weight cut-off 10 kDa), ultrafiltration stirred units and 0·22 μm filters were purchased from Millipore. Human recombinant TNF-α and IL-1β were from BD Biosciences. Dulbecco's PBS, minimal essential medium, l-glutamine and penicillin/streptomycin were obtained from Invitrogen. Fetal bovine serum was from Wisent. Antibodies (rabbit polyclonal anti-TLR4 IgG and Alexa Fluor 488 anti-rabbit goat antibody) were purchased from Santa Cruz Biotechnologies. All other chemicals were purchased from Sigma-Aldrich and were of highest analytical grade.
Hyperbaric treatment of whey protein isolate
The WP isolate was dissolved in double-distilled water to obtain a 15 % solution (w/v) and pressurised with an Avure High Pressure Processing System model QFP 215L-600 (Avure Technologies). As pressures above 500 MPa are required to denature most WP(Reference Huppertz, Kelly and Fox31), one cycle of pressurisation at 550 MPa with 1 min holding time was carried out to produce the pressurised WP (pWP) isolate. Control native WP (nWP) underwent the same treatment with omission of the pressurisation step. Solubilised pWP and nWP were frozen overnight at − 80°C, freeze-dried, flushed with N2 and stored in powdered form at − 20°C until further use.
In vitro enzymatic digestion
Powdered pWP and nWP isolates were dissolved in double-distilled water warmed up to 37°C, pH 1·9, at a concentration of 3 mg/ml. First-stage digestion was carried out by incubation for 15 min with pepsin prepared in 0·01 m-HCl (enzyme:substrate ratio 1:200). Then, pH was adjusted to 7·4, and the second-stage digestion was conducted for 60 min using trypsin, chymotrypsin and peptidase (all prepared in phosphate buffer, pH 7·0; enzyme:substrate ratios 1:200, 1:87 and 1:120, respectively). Afterwards, the enzymes were inactivated by the addition of 10 M-NaOH (final pH 10·5).
Isolation of peptides
After digestion, pWP and nWP hydrolysates were subjected to ultrafiltration to remove high-molecular weight peptides, which are unlikely to be absorbed through the intestinal mucosa. A membrane filter with a molecular weight cut-off of 10 kDa (Millipore) was used in a stirred ultrafiltration membrane reactor (Amicon Ultrafiltration Cell, model 8050); the filtration was allowed to proceed at 4°C under a N2 gas pressure of 40 pounds per square inch (psi). The peptides obtained through in vitro digestion and filtration had an average molecular weight of less than 1 kDa (data not shown), which corresponds to the size of the peptides likely to be intestinally absorbed in vivo (Reference Nellans32, Reference Roberts, Burney and Black33).
Cell culture and experimental studies
Immortalised human respiratory cell lines utilised in the present study were CFTE29o-, which bears the most common CF mutation (ΔF508), and 1HAEo- (non-CF cell line). Both cell lines were a kind gift from Dr D. Gruenert (University of California, San Fransisco). Basal cell culture conditions were as described in our previous studies(Reference Dauletbaev, Lam and Eklove34, Reference Dauletbaev, Eklove and Mawji35). For viability and IL-8 production experiments, cells were seeded at a density of 5 × 105 cells/ml in twenty-four-well cell culture plates and grown for 24 h in minimal essential medium supplemented with l-glutamine, penicillin/streptomycin and 10 % heat-inactivated fetal bovine serum. The next day, cells became 100 % confluent and were pre-incubated for 1 h with pWP or nWP hydrolysates (0–1000 μg/ml) in antibiotic-free minimal essential medium supplemented with 2 % fetal bovine serum. Cells were then stimulated with LPS, TNF-α or IL-1β, as described in the Results section, along with a fresh preparation of hydrolysates. After incubation for indicated amounts of time, cell viability and IL-8 secretion were assessed as described below. For analysis of total glutathione, cells were seeded in 35 mm culture dishes, cultured and treated as above, and collected for glutathione analyses with a slight modification of our previous protocol(Reference Vogel, Cinatl and Dauletbaev36).
Cell viability
Cell viability was assessed using the MTT assay(Reference Mosmann37, Reference Dauletbaev, Rickmann and Viel38), based on the reduction of MTT reagent into purple formazan crystals by viable metabolically active cells. Briefly, cells were washed with PBS and incubated with MTT solution (0·5 mg/ml) for 3 h. The formazan crystals were then dissolved in lysis solution containing 0·4 M-HCl and 100 % isopropanol, and absorbances were measured at 540 nm. Backgrounds were assessed at 600 nm and subtracted from the 540 nm values. Cell viability was expressed as a percentage of untreated control.
IL-8 analysis
IL-8 secretion in cell-free supernatants was assessed with a commercial human IL-8 ELISA kit (BD Biosciences) according to the manufacturer's instructions.
Limulus amebocyte lysate assay
LPS activity was assessed by the chromogenic Limulus amebocyte lysate (LAL) assay, using a commercial kit (Endochrome). Briefly, pWP hydrolysates at concentrations 500–2000 μg/ml in endotoxin-free water were incubated with LPS (2·5 μg/ml) for 30 min. Thereafter, 50 μl of the LPS hydrolysate solution were placed into ninety-six-well microtitre plates with an equal volume of LAL reagent and incubated at 37°C for 7 min. An addition of 100 μl of chromogenic substrate solution and further incubation for 5 min led to the development of a yellow colour. The reaction was stopped by the addition of 20 % acetic acid, and absorbances were read on a microplate reader at 405 nm.
Analysis of Toll-like receptor 4 surface expression
Cells were seeded in 60 mm culture dishes and cultured until confluent, then treated with pWP hydrolysate (1000 μg/ml) and stimulated with LPS as above. After stimulation for 24 h, cells were lifted using 50 mm-EDTA, washed with PBS and incubated with blocking solution (PBS supplemented with 1 % bovine serum albumin) for 30 min. Cells were then washed, incubated with anti-TLR4 rabbit antibody for 1 h, washed again and incubated with Alexa Fluor 488-conjugated goat anti-rabbit antibody for 45 min. A FACScalibur flow cytometer coupled with CELLQuest software (BD Biosciences) was used to analyse cell fluorescence. Cell debris was excluded using forward and side scatter characteristics, and cell fluorescence documented in the FL1 (green) channel. Results are expressed as a percentage of cells with TLR4 surface expression.
Analysis of lipopolysaccharide binding
LPS binding to surface TLR4 was assessed with a modification of a published protocol(Reference Abate, Alghaithy and Parton39). Briefly, cells were seeded in 60 mm culture dishes and pretreated with 1000 μg/ml of pWP hydrolysate. Cells were lifted using 50 mm-EDTA, washed and incubated with 1000 μg/ml of hydrolysate and 2·5 μg/ml of FITC-LPS for 30 min at 4°C. FITC-LPS was dissolved in PBS (10 % fetal bovine serum) as a source of LPS-binding protein. After washing, cells were resuspended in PBS, and cell fluorescence was analysed with a FACScalibur flow cytometer. Results are expressed as a percentage of cells with bound FITC-LPS.
Analysis of antioxidant capacity of cell-free supernatants
CFTE29o- and 1HAEo- cells were incubated with pWP or nWP hydrolysates for 24 h, following which cell-free supernatants were collected. The antioxidant capacity of these supernatants was assessed using the ferric-reducing antioxidant power (FRAP) assay(Reference Benzie and Strain40). This assay is based on the reduction of the Fe3+–2,4,6-tripyridyl-S-triazine (TPTZ) complex to the ferrous (Fe2+) form. Briefly, FRAP reagent was prepared with sodium acetate buffer (300 mm), 2·5 ml TPTZ solution (10 mm in 40 mm-HCl) and 2·5 ml ferric chloride solution (20 mm in double-distilled water) in a 10:1:1 ratio, respectively. Cell-free supernatants were incubated with FRAP reagent for 60 min, at a 1:20 ratio. A standard curve was constructed with bovine serum albumin, and absorbances were measured at 593 nm.
Analysis of intracellular total glutathione concentration
Glutathione was quantified using the enzymatic kinetic assay, adopted for a Cobas Mira S chemistry analyser(Reference Lands, Grey and Smountas41). Reagent concentrations were from the previously published protocol(Reference Dauletbaev, Rickmann and Viel42). Protein content, measured using the bicinchoninic acid protein assay kit (Pierce) according to the manufacturer's instructions, was utilised to normalise glutathione levels.
Statistical analysis
Data are presented as means with their standard errors. At least three independent experiments were conducted for each study. For cell culture assays, results were compared by one- or two-way ANOVA for each cell line, with Tukey's post hoc test to determine statistically significant differences between the control and treatment groups. A P value of less than, or equal to, 0·05 was considered as significant. Statistical analyses were performed using Sigma Stat version 2.03 (Systat Software, Inc.).
Results
Effects of whey protein hydrolysates on cell viability
We first verified that pWP or nWP hydrolysates are well tolerated over a wide range of concentrations by the respiratory epithelial cell lines. This was tested using the MTT assay, which showed that both pWP and nWP hydrolysates at concentrations of 12·5–1000 μg/ml were well tolerated by both cell lines (see Fig. S1, available online).
IL-8 secretion stimulated by lipopolysaccharide and TNF-α
The CF respiratory epithelium is exposed to bacterial products and pro-inflammatory cytokines. To exemplify these, CFTE29o- and 1HAEo- cells were incubated with LPS (2·5 μg/ml) and TNF-α (1 ng/ml) for 24 h to stimulate IL-8 secretion. Both stimuli significantly up-regulated IL-8 secretion in both cell lines; however, LPS was a weaker stimulus. Specifically, CFTE29o- and 1HAEo- cells up-regulated IL-8 in response to LPS by 3·8- and 5·7-fold, respectively, relative to their basal IL-8 secretion (P <0·05 v. basal, both cell lines; Fig. 1(a)). In comparison, TNF-α stimulation led to a 21·2 (CFTE29o-) and 10·0 (1HAEo-) fold increase in IL-8 secretion (P <0·05 v. basal, both cell lines; P< 0·01 v. LPS, both cell lines, data not shown). Since a 24 h exposure to TNF-α was overly hyperinflammatory, a shorter, 1 h exposure to this stimulus was tested. After the 1 h stimulation, the stimulus was removed, and cells were incubated for 23 h afterwards, at which point supernatants were collected for IL-8 ELISA. A shorter TNF-α stimulation resulted in a more modest IL-8 response, with CFTE29o- and 1HAEo- cells responding, respectively, more and less robustly than following 24 h exposure to LPS (CFTE29o-: 6·3-fold over basal; 1HAEo-: 2·1-fold over basal; P <0·05 v. basal, both cell lines; Fig. 1(b); P <0·05 v. stimulation with LPS). Although these IL-8 responses were still significantly different from those following LPS stimulation, they were within the same order of magnitude of the response elicited by LPS. Therefore, in subsequent experiments, potential suppressing effects of WP hydrolysates were tested in respiratory epithelial cells stimulated with LPS for 24 h and TNF-α for 1 h.

Fig. 1 Stimulation of IL-8 secretion in CFTE29o- (■) and 1HAEo- (□) cell lines. Cells were stimulated with (a) 2·5 μg/ml of lipopolysaccharide (LPS) for 24 h, (b) 1 ng/ml of TNF-α for 1 h followed by 23 h incubation in stimulus-free culture medium, (c) 50 pg/ml of IL-1β for 24 h, (d) 50 pg/ml of IL-1β for 1 h followed by 23 h incubation in stimulus-free culture medium. IL-8 concentrations were assessed in cell supernatants by ELISA. IL-8 secretion in stimulated cells was expressed as a fold increase over that of basal cells. Values are means of three or more independent experiments, with their standard errors represented by vertical bars. * Mean value was significantly different relative to basal IL-8 secretion (P <0·05).
Effects of whey protein hydrolysates on IL-8 secretion
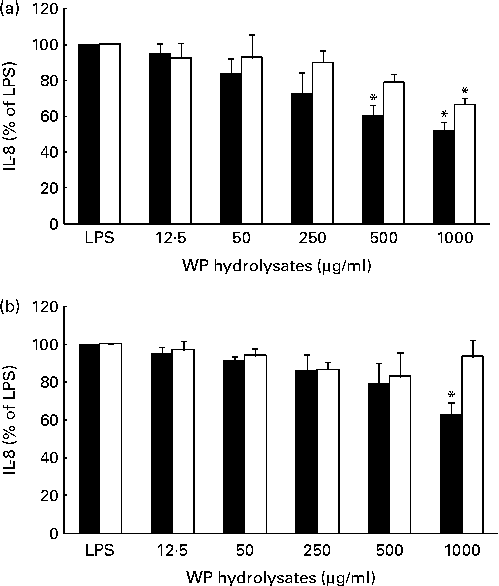
The potential anti-inflammatory effects of pWP and nWP hydrolysates were first tested in respiratory epithelial cells under unstimulated conditions. Basal secretion of IL-8 in either cell line was not significantly affected by pWP or nWP hydrolysates, up to a concentration of 1000 μg/ml, although a trend towards reduced secretion was noted with pWP hydrolysates (see Fig. S2, available online). The effects of WP hydrolysates on the stimulated production of IL-8 were then evaluated. In both cell lines, the up-regulation of IL-8 by LPS was partially reverted by pretreatment with WP hydrolysates, and more so with pWP hydrolysate (Fig. 2). Specifically, in CFTE29o- cells, 500 and 1000 μg/ml of pWP hydrolysate significantly suppressed the LPS-stimulated IL-8 by 40 and 48 %, respectively (P <0·05 v. LPS alone, both comparisons; Fig. 2(a)). In contrast, the suppression caused by nWP was more modest (only 34 % caused by nWP hydrolysate at a dose of 1000 μg/ml). While the latter decrease was still statistically significant (P <0·05 v. LPS alone), it also tended to be lower when compared with the suppression by pWP at a comparable dose (P= 0·07; 1000 μg/ml of nWP+LPS v. 1000 μg/ml of pWP+LPS).

Fig. 2 Effect of pressurised whey protein (WP, ■) and native WP (□) hydrolysates on lipopolysaccharide (LPS)-induced IL-8 secretion in (a) CFTE29o- and (b) 1HAEo- cell lines. Cells were pre-incubated with either hydrolysate for 1 h, and then stimulated with LPS (2·5 μg/ml) in the presence of fresh WP hydrolysates for 24 h. IL-8 concentrations were assessed in cell supernatants by ELISA. IL-8 secretion in cells treated with WP hydrolysates is expressed as a percentage of that in cells stimulated with LPS. Values are means of three to five independent experiments, with their standard errors represented by vertical bars. * Mean value was significantly different from that of LPS alone (P <0·05).
A qualitatively similar trend was observed in 1HAEo- cells. Specifically, incubation with 1000 μg/ml of pWP hydrolysate significantly suppressed the LPS-stimulated IL-8 (P <0·05 v. LPS alone), while incubation with 1000 μg/ml of nWP hydrolysate did not lead to a similarly significant suppression (Fig. 2(b)). Thus, experiments from both cell lines indicated that pWP hydrolysate exhibits a more potent suppressive ability towards the LPS-induced IL-8 response.
We also tested whether the IL-8-suppressing effects of WP hydrolysates would also extend to TNF-α-induced IL-8 secretion. In marked contrast to the LPS-stimulated IL-8, neither WP hydrolysate suppressed TNF-α-induced IL-8 secretion in either cell line (see Fig. S3, available online).
As the LPS and TNF-α pathways for stimulating IL-8 secretion converge towards the activation of the kinases leading to NF-κB activation (Fig. 3), we hypothesised that the suppression of IL-8 stimulated by LPS, and the lack of suppression towards TNF-α stimulation, were due to the effects of WP hydrolysates upstream of NF-κB activation. The higher anti-IL-8 potency of pWP could also be exerted by a more potent effect on that particular segment of the IL-8 pathway.

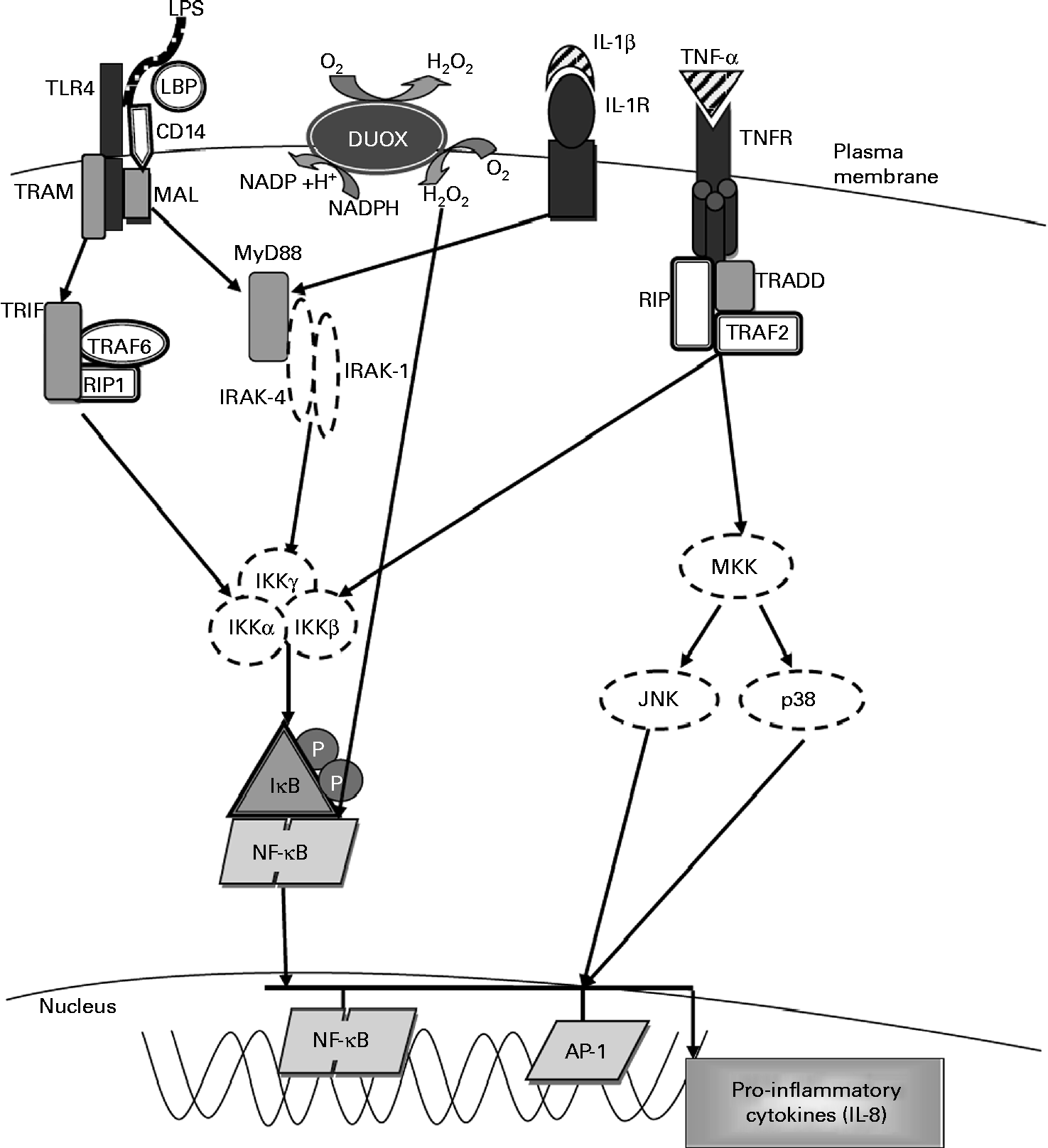
Fig. 3 Molecular pathways leading to IL-8 expression. The activation of Toll-like receptor 4 (TLR4) by lipopolysaccharide (LPS) involves the binding of the LPS–LPS-binding protein (LBP) complex to CD14, transferring LPS to MD-2 and TLR4. This results in the recruitment of the adaptor proteins myeloid differentiation factor (MyD88) and MyD88 adaptor-like (MAL). MyD88 associates with the IL-1 receptor-associated kinases (IRAK) and TNF receptor-associated factor-6 (TRAF6) and dissociates from the TLR4 complex. This results in the activation of the inhibitory-κB kinase (IKK) complex, which catalyses IκBα phosphorylation and subsequent degradation, thereby allowing NF-κB to translocate into the nucleus, where it activates the gene transcription of IL-8 and other pro-inflammatory cytokines. Exposure to LPS also leads to the activation of dual oxidase 1 (DUOX1) and the subsequent generation of hydrogen peroxide leading to NF-κB activation. Stimulation with IL-1β through the IL-1 receptor (IL-1R) activates the same MyD88-dependent pathway as associated with TLR4. Exposure to TNF-α leads to NF-κB activation via the TNF receptor (TNFR), which recruits the adaptor protein TNFR-associated death domain (TRADD). TRADD associates with additional adaptor proteins, TNFR-associated factor 2 (TRAF2) and receptor-interacting protein (RIP), initiating a signalling cascade that leads to NF-κB activation. TRAM, TRIF-related adaptor molecule; TRIF, Toll-IL-1 receptor domain-containing adaptor inducing interferon β; MKK, mitogen-activated protein Kinase; JNK, c-Jun N-terminal kinase; Ap-1, activated protein-1. Adapted from Nakanaga et al. (Reference Nakanaga, Nadel and Ueki45), Boots et al. (Reference Boots, Hristova and Kasahara46), Verstrepen et al. (Reference Verstrepen, Bekaert and Chau50) and Greene & McElvaney(Reference Greene and McElvaney66).
The LPS pathway upstream of NF-κB-activating kinases, which is the presumed target for WP hydrolysates, overlaps with the IL-1β pathway at MyD88, i.e. immediately after extracellular ligand binding to their respective receptors (Fig. 3). Should the anti-IL-8 effects of WP hydrolysates occur at the level or downstream of MyD88, then WP hydrolysates would suppress IL-8 stimulated by IL-1β. To test this, and to begin elucidating the possible mechanisms by which WP hydrolysates exert their anti-inflammatory effect, the following studies were devised.
Since we observed that 24 h stimulation with TNF-α leads to the overproduction of IL-8, we first tested IL-8 responses from cells stimulated with 1 ng/ml of IL-1β for 1 or 24 h. As in our previous studies(Reference Dauletbaev, Lam and Eklove34, Reference Dauletbaev, Eklove and Mawji35), we observed that IL-1β stimulation results in a very robust up-regulation of IL-8 secretion in CFTE29o- cells (71·8- and 13-fold for the 24 and 1 h exposure, respectively, data not shown) and in 1HAEo- cells (51·5- and 12·3-fold, respectively, data not shown). Subsequently, the dose of IL-1β was decreased to 50 pg/ml in order to elicit a response comparable with that obtained with LPS stimulation. The 24 h exposure of CFTE29o- and 1HAEo- cells to 50 pg/ml of IL-1β resulted, respectively, in a 7·9- and 21·1-fold increase in IL-8 production (P <0·05 v. basal; Fig. 1(c); P <0·05 v. stimulation with LPS). In contrast to stimulation with LPS, neither WP hydrolysate, applied as above, affected IL-1β-induced IL-8 secretion (see Fig. S4, available online). We also tested whether WP hydrolysates would be more effective against the 1 h exposure to 50 pg/ml of IL-1β. The 1 h stimulation was followed by the removal of IL-1β and the incubation of cells for 23 h in the stimulus-free culture medium, which, similar to a shorter TNF-α stimulation, led to a less pronounced increase in IL-8 secretion (2·4- and 4·2-fold in CFTE29o- and 1HAEo- cells, respectively; P <0·05 v. basal; Fig. 1(d); P>0·1 v. stimulation with LPS). Still, WP hydrolysates were not effective against this weaker IL-1β stimulation (see Fig. S4, available online). This suggested that the effect of WP hydrolysates on LPS-stimulated IL-8 secretion was either due to the direct interaction with LPS, or occurred at the level of LPS binding to TLR4, the cellular sensor for LPS. In order to explore this hypothesis, the effects of WP hydrolysates on LPS activity, cell surface TLR4 expression and LPS–TLR4 binding were studied. In some of these experiments, only pWP was tested as the more potent of the whey hydrolysates.

Fig. 4 Effect of (a) pressurised and (b) native whey protein (WP) hydrolysates on lipopolysaccharide (LPS) binding to surface Toll-like receptor 4 in CFTE29o- and 1HAEo- cell lines. Cells were pretreated for 1 h with WP hydrolysates (1000 μg/ml), detached and incubated with 1000 μg/ml of WP hydrolysates and 2·5 μg/ml of flurescein isothiocyanate (FITC)-LPS for 30 min at 4°C, washed and resuspended in PBS for flow cytometric analysis. Data are expressed as a percentage of cells stimulated with LPS only. Values are means of four independent experiments, with their standard errors represented by vertical bars. * Mean value was significantly different from that of LPS alone (P <0·05; paired t test).
Lipopolysaccharide-neutralising activity of pressurised whey protein hydrolysate
Potential direct LPS-neutralising activity of WP was tested using a quantitative chromogenic LAL assay. Inhibition of LPS-induced LAL activation by WP hydrolysates would indicate binding and neutralisation of the biological effects of LPS. No inhibition of LPS activity by 1000 μg/ml of pWP hydrolysate was observed, ruling out a direct interaction between WP hydrolysates and LPS (see Fig. S5, available online). Further, even a 2000 μg/ml concentration of the hydrolysate did not inhibit LPS-induced activation of the LAL (see Fig. S5, available online).
Effect of whey protein hydrolysates on the surface expression of Toll-like receptor 4
We next evaluated whether WP hydrolysates would affect TLR4 expression on the cell surface, as the down-regulation of TLR4 is likely to affect the magnitude of the LPS-stimulated IL-8 response(Reference MacRedmond, Greene and Dorscheid43). Flow cytometry was used to assess the surface expression of TLR4 in response to 24 h stimulation with LPS alone or in combination with pWP hydrolysate (1000 μg/ml). Neither LPS nor its combination with pWP hydrolysate affected surface TLR4 expression in either cell line (see Fig. S6, available online).
Effect of whey protein hydrolysates on lipopolysaccharide binding to surface Toll-like receptor 4
As neither LPS activity nor TLR4 cell surface expression was affected by pWP hydrolysate, we next examined the effect of WP hydrolysates on LPS binding to TLR4. Cells were pretreated for 1 h with pWP or nWP hydrolysates (1000 μg/ml), and the ability of FITC-labelled LPS to bind to cell surface TLR4 was assessed by flow cytometry. Treatment with pWP hydrolysate resulted in a significant reduction in cell surface-bound FITC-LPS in both CFTE29o- and 1HAEo- cells (27·3 and 31·5 %, respectively; P <0·05 v. LPS alone; Fig. 4(a)). Treatment with nWP hydrolysates also resulted in a similar magnitude of decrease (31·1 % in CFTE29o- and 31·7 % in 1HAEo- cells; P <0·05 v. LPS alone; Fig. 4(b)).
Effect of whey protein hydrolysates on the antioxidant capacity of cell culture medium
The mechanistic experiments, presented above, only partially explained the higher anti-IL-8 potency of pWP hydrolysate. Whey is a rich source of peptides with antioxidant properties presumably revealed to a greater extent by pressurisation(Reference Zavorsky, Kubow and Grey29). These antioxidant peptides may diminish the oxidative signalling which is a part of inflammatory signalling cascades(Reference Rahman and MacNee3). Therefore, we next hypothesised that pWP hydrolysate might exhibit a greater antioxidant capacity. The latter hypothesis was tested in subsequent experiments at two levels, as enhancement of extracellular antioxidant capacity and as potentiation of intracellular antioxidant content.
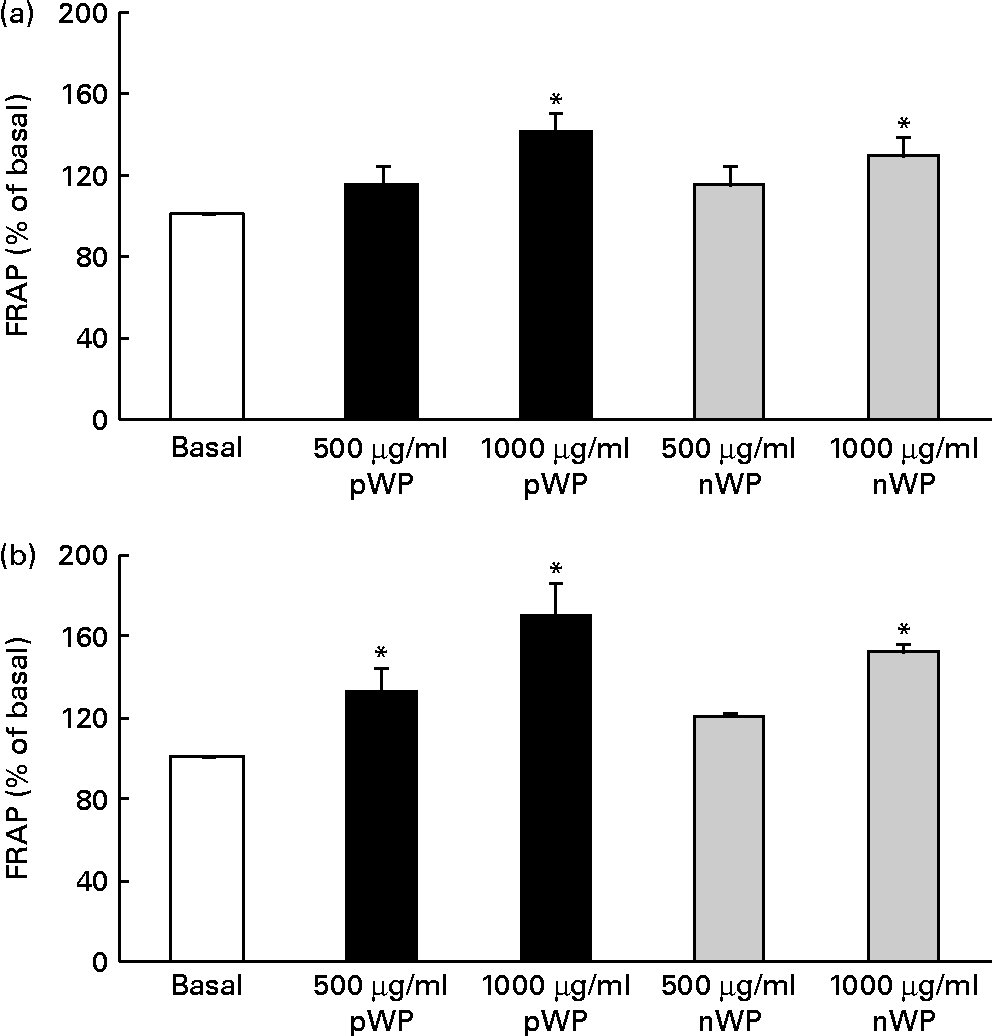
Respiratory epithelial cells express a homologue of NADPH oxidase, dual oxidase (DUOX) 1, which has been implicated in inflammatory responses via its role in the generation of extracellular H2O2(Reference Fischer44). It has also been shown that DUOX1 up-regulates IL-8 expression by generating intracellular H2O2 and reactive oxygen species (ROS)(Reference Nakanaga, Nadel and Ueki45, Reference Boots, Hristova and Kasahara46). We therefore asked whether WP hydrolysates might be interfering with DUOX1-induced ROS production, thereby affecting IL-8 secretion, and whether pWP hydrolysate would exhibit a greater ROS-suppressing potential. We first attempted to quantify intracellular ROS production directly in cells supplemented with WP hydrolysates. Unfortunately, this measurement was not reliable due to high inter-well variability (data not shown). To circumvent this limitation, we then evaluated extracellular H2O2 production indirectly, using the FRAP assay of cell culture medium following the 24 h exposure of cells to pWP and nWP hydrolysates. In both cell lines, treatment with pWP and nWP hydrolysates (500 or 1000 μg/ml each) resulted in a dose-dependent increase in FRAP, suggesting an enhanced H2O2-scavenging capacity of extracellular microenvironment (Fig. 5). In both CFTE29o- and 1HAEo- cell lines, pWP hydrolysate tended to increase this antioxidant capacity of the cell culture medium more potently than nWP, although this did not reach statistical significance (Fig. 5).

Fig. 5 Effect of pressurised whey protein (pWP) and native whey protein (nWP) hydrolysates on cell culture medium ferric-reducing antioxidant power (FRAP). (a) CFTE29o- and (b) 1HAEo- cell lines were treated for 24 h with 500 or 1000 μg/ml of pWP (■) or nWP (![]() ) hydrolysates, following which extracellular antioxidant capacity was assessed using the FRAP assay. Data are expressed as a percentage of basal. Values are means of five independent experiments, with their standard errors represented by vertical bars. * Mean value was significantly different relative to basal (P <0·05).
) hydrolysates, following which extracellular antioxidant capacity was assessed using the FRAP assay. Data are expressed as a percentage of basal. Values are means of five independent experiments, with their standard errors represented by vertical bars. * Mean value was significantly different relative to basal (P <0·05).
Effect of lipopolysaccharide and/or whey protein hydrolysates on intracellular total glutathione concentration
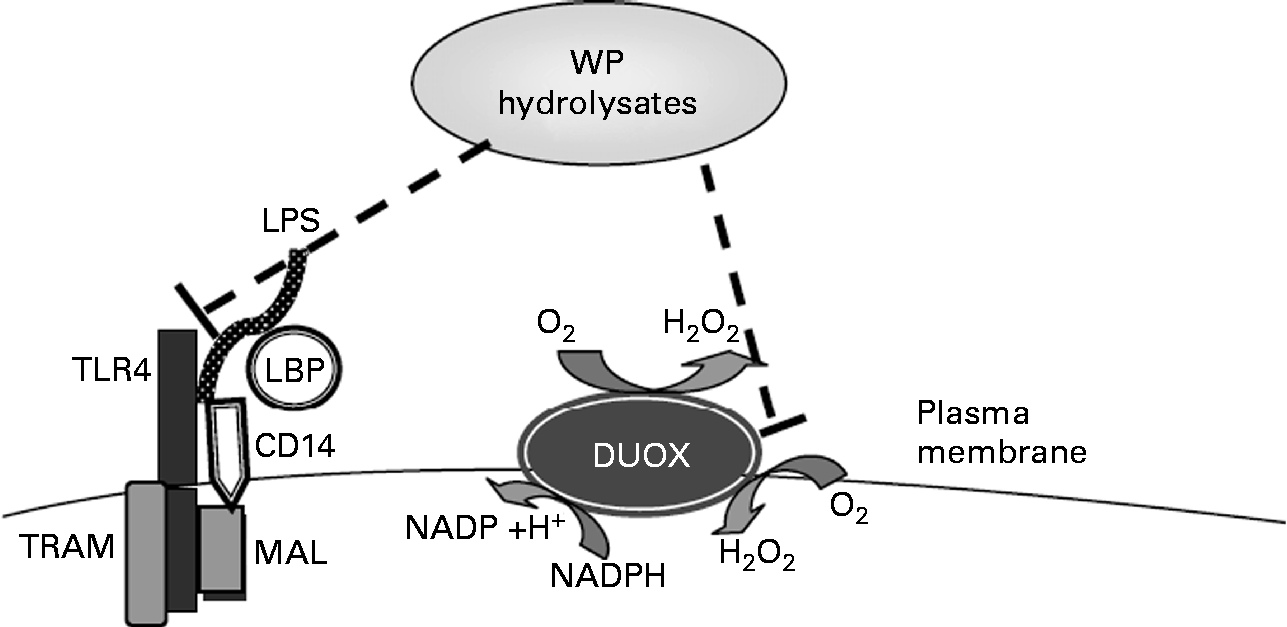
Whey hydrolysates may also increase the intracellular antioxidant status and thus inhibit stimulated IL-8 responses (Fig. 6). To examine a potential effect of WP hydrolysates on changes in intracellular antioxidant status, intracellular total glutathione concentrations were studied. Whey, a mixture of cysteine-rich proteins, has been shown to affect intracellular levels of glutathione(Reference Kent, Harper and Bomser47), a crucial low-molecular weight antioxidant, which, in turn, has been reported to influence oxidant-sensitive pro-inflammatory transcription factors(Reference Rahman and MacNee48). Neither LPS nor WP hydrolysates had any significant effect on intracellular total glutathione concentrations (data not shown).

Fig. 6 Tentative molecular mechanisms by which whey protein (WP) hydrolysates lead to the suppression of lipopolysaccharide (LPS)-stimulated IL-8 secretion. TLR4, Toll-like receptor 4; LBP, LPS-binding protein; DUOX, dual oxidase; TRAM, TRIF-related adopted molecule; TRIF, Toll-IL-1 receptor-domain-containing adaptor inducing interferon β; MAL, MyD88 adaptor-like.
Discussion
The major original finding from the present study is that in vitro pretreatment of CF and non-CF respiratory epithelial cells with low-molecular weight peptide and amino acid products ( < 1 kDa) from WP digestion significantly suppresses LPS-induced IL-8 secretion. The present findings are a first demonstration that whey-derived peptides can down-regulate the LPS-induced inflammatory response, which adds to previous findings showing the immunomodulatory effects of WP in animal models(Reference Beaulieu, Girard and Dupont19, Reference Hayashida, Kaneko and Takeuchi21, Reference Shimizu, Dairiki and Ogawa24) and in vitro (Reference Otani, Horimoto and Monnai22, Reference Otani and Monnai49).
A key observation in the present study was that IL-8 secretion was suppressed by the WP hydrolysates following stimulation with LPS but not with TNF-α or IL-1β. The binding of LPS and TNF-α to their respective receptors, TLR4 and TNF receptor, results in two distinct signalling cascades which lead to the activation of transcription factor NF-κB and IL-8 release. In addition, activation of the IL-1β receptor leads to an intracellular signalling cascade shared by both IL-1β receptor and TLR4(Reference Verstrepen, Bekaert and Chau50). Since there was no effect of WP hydrolysates on TNF-α or IL-1β-induced IL-8 secretion, this suggests that WP hydrolysates exert their effects upstream of MyD88, i.e. at the receptor level. Further investigation ruled out a possible effect of WP hydrolysates on cell surface TLR4 expression. Previous studies have demonstrated contradictory findings in terms of the possible induction of TLR4 surface expression by LPS, with some authors reporting an up-regulation(Reference John, Yildirim and Rubin51) and others finding no effect(Reference Muir, Soong and Sokol52). We did not see any effect of LPS or WP hydrolysates on TLR4 cell surface expression, and differences from previous reports can be attributed to tissue specificity and differences in the cell culture conditions employed.
As some food-derived peptides may interact with cell surface receptors(Reference Foltz, Ansems and Schwarz53, Reference Kitazawa, Yonezawa and Tohno54), we deemed it plausible that inhibition of LPS activity by WP hydrolysates could involve the inhibition of TLR4 activation. Indeed, the present results show that binding of LPS to TLR4 was decreased in the presence of WP hydrolysates. Lee et al. (Reference Lee, Farmer and Hilty20) proposed that a direct inhibition of the binding of LPS to monocytes by lactoferrin is the mechanism by which lactoferrin feeding of neonatal piglets exerted anti-inflammatory effects following intravenous exposure to LPS. A variety of anti-bacterial peptides including whey-derived peptides have been shown to neutralise LPS via direct binding to the lipid A portion of LPS(Reference Zhang, Mann and Tsai55). Since the lipid A portion of LPS is responsible for both TLR and LAL activation(Reference Iwanaga, Miyata and Tokunaga56, Reference Rietschel, Kirikae and Schade57), the lack of the effect of WP hydrolysates on LAL activation indicates that WP hydrolysates do not inactivate LPS. Taken together, our data suggest that the inhibitory effect of WP hydrolysates was most probably due to a direct interaction of the WP hydrolysates with the receptor, thereby preventing LPS recognition. We cannot eliminate, though, that WP hydrolysates also interfere with the recognition of LPS by CD14 or LPS-binding protein, events which are necessary for TLR4 activation.
Another possible mechanism by which WP hydrolysates may have modulated the inflammatory response is via improved antioxidant status either by provision of hydrogen-donating peptides or by contributing amino acids with antioxidant capacity, such as cysteine, tyrosine or tryptophan(Reference Pihlanto58). Redox status is a known modulator of the inflammatory response(Reference Rahman and MacNee3), as oxidative stress up-regulates the production of inflammatory cytokines in vitro (Reference Gutierrez-Ruiz, Gomez Quiroz and Hernandez59). WP have antioxidant properties in vivo (Reference Pihlanto58, Reference Elia, Stadler and Horvath60), and whey-derived peptides have been shown to exert free radical-scavenging activities both in vitro (Reference Hernandez-Ledesma, Davalos and Bartolome61) and in vivo (Reference Elia, Stadler and Horvath60). Moreover, the NADPH oxidase homologue DUOX1, in addition to its role in host defence via generating extracellular H2O2(Reference Van der Vliet62), has been attributed a role in cell signalling resulting in IL-8 secretion. It has also been shown that ROS production by DUOX1 is necessary for such signalling to occur(Reference Nakanaga, Nadel and Ueki45). In that regard, we assessed the antioxidant capacity of cell culture medium following treatment of cells with WP hydrolysates, as an indirect indicator of extracellular H2O2 production. The present results show that WP hydrolysates significantly increased the antioxidant capacity of cell culture medium as assessed by the FRAP assay, suggesting an additional mechanism by which WP hydrolysates may exert their IL-8-suppressing effects. This antioxidant effect was somewhat enhanced with pressurisation of whey.
We did not find any effect of either LPS or WP hydrolysates on intracellular total glutathione concentrations. It is possible that either LPS or WP hydrolysates could have altered the relative proportions of oxidised and reduced glutathione, without affecting total glutathione concentrations, or altered intracellular redox status. These measurements would have contributed to a clearer understanding of these potential mechanisms. Nonetheless, we speculate that the superior effect of pWP hydrolysates in suppressing LPS-stimulated IL-8 responses is due to a combination of interference with LPS binding to TLR4 and altered redox status.
Previous results(Reference Vilela, Lands and Chan28) demonstrate that pressurisation results in qualitatively different peptide profiles from those obtained from native whey digestion as opposed to an increased number of available peptides. As pressurisation of WP potentiated the inhibitory effect of hydrolysates on the IL-8 response to LPS, as well as the enhancement of cell culture medium antioxidant capacity, this probably reflects a profile of relatively greater amounts of immunomodulatory and antioxidant peptides generated from the hydrolysis of pressurised WP. In that regard, hyperbaric pressure treatment of WP induces changes in their secondary and tertiary structures, exposing hidden and otherwise unavailable peptide sequences to enzymatic digestion. Indeed, WP are highly resistant to enzymatic digestion(Reference Reddy, Kella and Kinsella63), and undigested WP (namely β-lactoglobulin, α-lactoglobulin, Ig and lactoferrin) have been found in the intestinal lumen(Reference Walzem, Dillard and German64). Increasing the susceptibility of WP to digestion via pressurisation may alter the spectrum of absorbable peptides to increase the availability of bioactive peptides for intestinal absorption.
To our knowledge, the present findings demonstrate for the first time that whey-derived peptides could have an impact on inflammatory responses involving LPS-mediated IL-8 release and adds to clinical work showing a trend to IL-8 down-regulation with pressurised whey supplementation in CF patients, in addition to enhanced nutritional status(Reference Lands, Iskandar and Beaudoin30). There is increasing evidence that Gram-negative bacterial infections that lead to LPS-induced IL-8 release can exacerbate the inflammatory responses in chronic lung diseases such as CF(Reference Watt, Courtney and Moore65) and thereby contribute to lung pathophysiology. Although more studies are needed, the present study provides new insight and opens new avenues of research into the potential utilisation of WP in pro-inflammatory conditions involving the overproduction of IL-8 such as CF, particularly in relation to bacterial-induced inflammation. Effective suppression of IL-8 overproduction is a valid therapeutic target in CF(Reference Konstan and Davis11). Oral corticosteroids have unacceptable adverse side effects, inhaled corticosteroids have yet to prove long-term efficacy and high-dose ibuprofen has not gained popularity due to its varying bioavailability and potential adverse effects(Reference Dinwiddie12, Reference Lands and Dauletbaev13). The IL-8-suppressing effects of WP hydrolysates demonstrated herein add an interesting possible therapeutic dimension to WP as a nutrition-based adjuvant to conventional therapy towards the restoration of a homeostatic anti-inflammatory balance in CF.
In conclusion, we demonstrate that hydrolysates of WP suppress LPS-stimulated IL-8 secretion in vitro by affecting LPS binding to its receptor, TLR4, and by promoting enhanced extracellular antioxidant capacity. Pressurisation of whey tends to potentiate the latter mechanism, leading to a higher IL-8-suppressing activity.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114512004655
Acknowledgements
The authors thank Brian Meehan for his technical help. M. M. I. was sponsored by a doctoral studentship award from the Research Institute of the Montreal Children's Hospital. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. M. M. I. performed all in vitro digestions, ultrafiltrations, cell culture experiments, endpoint assays, data and statistical analyses, and drafted the manuscript. N. D. performed the glutathione and flow cytometric measurements, provided substantial advice with study design and contributed to the writing of the manuscript. S. K. contributed to the concept and study design, the data analysis and the writing of the manuscript. N. M. helped with replicating and troubleshooting the in vitro digestion experiments, and performed a subset of endpoint assays (FRAP, ELISA). L. C. L. contributed to the concept and study design, the data analysis and the writing of the manuscript. The authors declare no conflict of interest.