Introduction
Tau is a phosphoprotein that belongs to the microtubule-associated family (MAP). In the human brain, tau proteins can assume six different isoforms, with each variant containing a microtubule-binding domain comprising three repeat (3R) or four repeat (4R) regions in the protein carboxy-terminal (C-terminal) as well as one or two amino acid terminal (N-terminal) inserts.Reference Buee, Bussiere, Buee-Scherrer, Delacourte and Hof 1 Importantly, the expression of tau isoforms may not be equal across neurons. For instance, tau messenger RNAs containing exon 10—which encodes the fourth microtubule-binding domain—are not found in granular cells within the dentate gyrus.Reference Goedert, Spillantini, Jakes, Rutherford and Crowther 2 As such, tau isoforms are thought to be differentially distributed within neuronal subpopulations.
A key mediator of microtubule assembly and stability,Reference Weingarten, Lockwood, Hwo and Kirschner 3 - Reference Horio and Hotani 5 tau functionality is regulated by a wide range of serine and threonine phosphorylation sites. In addition to reducing tau’s affinity for microtubules, abnormal phosphorylation of these sites results in a complementary toxic gain of function—in the form of an increased propensity for misfolding and subsequent polymerization—with this mechanism believed to occupy a central role in various neurodegenerative conditions,Reference Jucker and Walker 6 , Reference Spires-Jones, Stoothoff, de Calignon, Jones and Hyman 7 including Alzheimer’s disease (AD), and the tau molecular subgroup of frontotemporal lobar degeneration (FLTD-tau) (Table 1). In the case of AD, the accumulation of abnormally phosphorylated tau is known to proceed hierarchically—affecting first the transentorhinal pre-α layer (Braak stage I–II), before progressing toward limbic (Braak stage III/IV) and isocortical areas (Braak stage V/VI)—with its spread thought to occur following a shift toward prion-like self-propagation.Reference de Calignon, Polydoro and Suarez-Calvet 8 , Reference Braak and Braak 9
Table 1 Tau isoforms in AD and various non-AD tauopathies

3R=three repeat tau microtubule binding domains; 4R=four repeat tau microtubule binding domains; AD=Alzheimer’s disease.
Though quantitative assessment of cerebrospinal fluid (CSF) levels of tau and phosphorylated tau (p-tau) are currently acknowledged as biomarkers of neurodegeneration,Reference Jack, Knopman and Jagust 10 with levels shown to correlate with cognitive impairment in AD,Reference Shaw, Vanderstichele and Knapik-Czajka 11 - Reference Augustinack, Schneider, Mandelkow and Hyman 13 the collection of CSF via lumbar puncture is invasive in nature and is associated with high inter-laboratory variability, hampering comparison of data across centers.Reference Mattsson, Blennow and Zetterberg 14 In addition, levels of tau and p-tau are unable to provide information regarding the topography of tau pathology in the brain, which is critical to the differential diagnosis of certain tauopathies.Reference Fodero-Tavoletti, Okamura and Furumoto 15 , Reference Spies, Claassen, Slats, Olde Rikkert, Verbeek and Kessels 16 The concept of misfolded tau as a central process underlying neurodegenerationReference Goedert and Jakes 17 - Reference Roberson, Scearce-Levie and Palop 20 has led to the development of tau-focused therapeutics aiming to reduce tau-mediated neurodegeneration, with approaches including microtubule stabilizing agents, reduction of tau hyperphosphorylation, inhibition of tau fibril aggregation, and promotion of microtubule stability.Reference Giacobini and Gold 21 As such, the development of noninvasive methodologies has a high priority for determining tau pathology propagation and monitoring treatment effects in clinical trials.
Positron emission tomography (PET), a noninvasive molecular imaging method, allows for quantitative analysis of a wide array of biological processes in the living human brain. Recently, three new classes of radiopharmaceuticals with a high affinity for tau tangles have been described (Table 2). PET using tau radiopharmaceuticals holds the promise of accurate, reliable, and reproducible quantification of both regional and global tau burden, which could translate into earlier and more accurate differential diagnosis, as well as aid in monitoring disease progression and therapeutic efficacy in clinical trials.
Table 2 Tau Radiopharmaceuticals

KD=ligand property equal to the inverse of affinity
In a recent review, we underscored the opportunity for improved modeling using tau radiopharmaceuticals and animals models.Reference Zimmer, Leuzy, Bhat, Gauthier and Rosa-Neto 26 Here, we shed light on the most promising tau radiopharmaceuticals and highlight future directions for tracking tau pathology using PET molecular imaging.
Tau Radiopharmaceuticals
Until recently, the main focus of PET molecular imaging has been the development of highly specific ligands for early detection of amyloid deposition. A variety of such tracers, including Pittsburgh compound B ([11C]PiB) and several [18F]-labeled tracers, have been developed and have provided important new insights into the role played by amyloid deposition in neurodegenerative disorders.Reference Rowe, Ng and Ackermann 27 Though one tracer among these [18F]FDDNP, appeared to bind both amyloid plaques and tau tangles,Reference Agdeppa, Kepe and Liu 28 a subsequent study using [3H]FDDNP autoradiography in sections containing neurofibrillary tangles (NFTs) failed to demonstrate overt labeling of tau pathology because of a low affinity for NFTs.Reference Thompson, Ye and Morgenstern 29 In fact, low levels of tau aggregates in the brain (relative to β-amyloid), as well as the multiple structural conformations it can assume, make tau tracking a more complicated task when compared with detection of β-amyloid deposition.Reference Mukaetova-Ladinska, Harrington, Roth and Wischik 30
Recently, three new classes of compounds have been developed aiming to bind tau fibrils and stand as potential tau imaging biomarkers. These include: (1) quinoline derivatives, (2) benzimidazole pyrimidine derivatives, and (3) benzothiazole derivatives.Reference Fodero-Tavoletti, Okamura and Furumoto 15 , Reference Xia, Arteaga and Chen 23 , Reference Maruyama, Shimada and Suhara 25 In the following paragraphs, we describe the most important insights generated from in vitro and in vivo studies using these novel tracers (for detailed information, see Table 3).
Table 3 Most relevant in vitro and in vivo studies conducted with tau radiopharmaceuticals
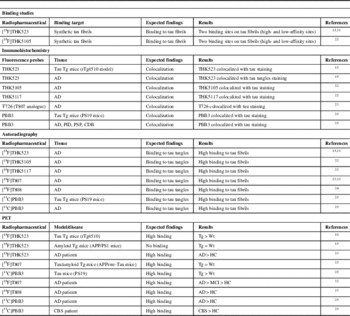
AD=Alzheimer’s disease; HC=healthy control; CBS=corticobasal syndrome; MCI=mild cognitive impairment; PID=Pick’s disease;
PSP=progressive supranuclear palsy; Tg=transgenic; Wt=wild-type.
Quinoline Derivatives
The first quinoline derived tau ligand was [18F]THK523. Following optimization in the form of improved specificity, second-generation tracers were released in the form of [18F]THK5105 and [18F]THK5117. In vitro binding assays involving such tracers were performed using synthetic truncated tau (K18ΔK280) fibrils, comprising four repeat regions (244-372) in the absence of lysine 280 (ΔK280). K18ΔK280 tau aggregates form rapidly without cofactors and exhibit similar characteristics to paired helical filamentous (PHF) tau.Reference Barghorn, Davies and Mandelkow 34 , Reference von Bergen, Barghorn and Muller 35 These studies demonstrated that [18F]THK5105 and [18F]THK5117 are associated with higher binding for K18ΔK280 tau aggregates, relative to [18F]THK523.Reference Okamura, Furumoto and Harada 22 Using histofluorescence analysis, Fodero-Tavoletti and colleaguesReference Fodero-Tavoletti, Okamura and Furumoto 15 showed that [18F]THK523 colocalizes with tau tangles and presents negligible binding to β-amyloid plaques in hippocampal tissue obtained from AD patients. Furthermore, they showed colocalization of [18F]THK523 and tau tangles in mouse (Tg4510, tau model) tissue immunostained with tau antibodies. In contrast, there was no colocalization in a mouse model harboring human amyloid precursor protein (APP) and presenilin 1 (PS1) mutations, a model that displays amyloidosis, but not tau tangles. [18F]THK523 bound to tau aggregates in AD hippocampal sections and not to amyloid pathology confirmed using anti-β-amyloid and anti-tau antibodies.Reference Fodero-Tavoletti, Okamura and Furumoto 15 Furthermore, [18F]THK523 was found to accumulate in Sommer’s sector (CA1) as well in layers pre- and pri-α of the entorhinal cortex in AD brain sections. These findings were consistent with the density of PHF-tau deposition, as confirmed via immunohistochemistry.Reference Harada, Okamura and Furumoto 31 However, a recent autoradiography study showed that [18F]THK523 has affinity only for tau filaments present in AD brains, but not for tau lesions present in non-AD tauopathies.Reference Fodero-Tavoletti, Furumoto and Taylor 36 Immunostaining with fluorescent THK5105 and THK5117 indicated colocalization with tau (AT8 antibody) in human hippocampal brain sections.Reference Okamura, Furumoto and Harada 22 In addition, autoradiography using [18F]THK5117 showed strong accumulation in regions displaying high amounts of tau deposition, such as the subiculum, parahippocampus, CA1 subfield, insula, inferior and middle temporal gyri, and the cingulate gyrus.Reference Okamura, Furumoto and Harada 22 Importantly, in vitro validation using binding assays and autoradiography combined with immunohistochemistry is sine qua non for the development of novel radiopharmaceuticals. In fact, these parameters are used to define which radiopharmaceuticals should be further investigated in in vivo studies.
In vivo studies showed that [18F]THK523, [18F]THK5105, and [18F]THK5117 exhibited sufficient amounts of tracer uptake in the mouse brain after intravenous infusion, although [18F]THK5105 and [18F]THK5117 showed higher brain uptake and faster clearance than [18F]THK523.Reference Okamura, Furumoto and Harada 22 Though microPET assessment using [18F]THK523 showed higher retention in rTg4510 mice relative to APP/PS1 mice and wild-type littermates, the first clinical PET study revealed elevated white matter (WM) retention, precluding future use of [18F]THK523 in research or clinical settings.Reference Villemagne, Furumoto and Fodero-Tavoletti 33 In the case of [18F]THK5105, however, unpublished preliminary data (courtesy of professors Okamura and Villemagne) revealed labeling of tau pathology in a subject with AD, with delineated areas (e.g. hippocampus) differing from those identified using [11C]PiB (Figure 1).

Figure 1 Co-registered MRI, [11C]PiB and [18F]THK-5105 images in a patient with Alzheimer’s disease (AD). Figure depicts a structural MRI (left), [11C]PIB (center), and [18F]THK-5105 PET images (right), from a patient with dementia resulting from AD (images courtesy of Profs. V. Villemagne and N. Okamura). Images were co-registered in the plane encompassing the temporal lobe, hippocampus, orbitofrontal cortex, amygdala, midbrain, and cerebellum. MRI shows bilateral hippocampal atrophy (right>left). [11C]PIB images show high uptake (yellow-red spots) in the temporal neocortex. [18F]THK-5105 shows high uptake (yellow-red spots) in the mesial temporal as well as the temporal neocortex. The high [18F]THK-5105 uptake in the midbrain possibly represents high nonspecific binding, a finding that would prove consistent with the vast literature addressing AD imaging and neuropathology. Of note, low uptake of both [11C]PIB and [18F]THK-5105 can be seen in the cerebellum, an important finding because the cerebellum serves as the reference region when determining the standardized uptake value ratio (SUVR). A semiquantitative approach, SUVR is defined as the ratio of cortical to reference region radioactivity (i.e. tracer retention). In the case of [11C]PiB and [18F]THK-51505 here shown, the reference regions are the cerebellum (vermis excluded) and the pons, respectively.
Benzimidazole Pyrimidines
After screening more than 900 compounds, benzimidazole pyrimidines derivatives [18F]T807 and [18F]T808 were developed. For characterization, a T807 analogue (T726) was used by Xia and colleaguesReference Xia, Arteaga and Chen 23 and showed a high degree of colocalization with PHF-tau but not with Aβ1-42 in frontal lobe postmortem tissue exhibiting AD pathology.
Autoradiography using [18F]T807 was conducted on frontal brain sections in three different groups classified as high PHF-tau and β-amyloid (group A); low PHF-tau, high β-amyloid (group B); and negative PHF-tau/β-amyloid (group C). Although strong gray matter signals were detected in group A, cortical regions from group B emitted only weak signals, and group C emitted only background signal. Comparison between double immunostained adjoining brain sections and [18F]T807 autoradiography revealed that [18F]T807 signals colocalized with immunostaining for PHF-tau but not with Aβ1-42 plaques.Reference Xia, Arteaga and Chen 23 [18F]T807 autoradiographic analysis using frontal lobe sections provided a selectivity estimate of 29-fold for tau relative to β-amyloid, confirmed by immunohistochemistry.Reference Chien, Bahri and Szardenings 32 In agreement, autoradiography with [18F]T808 also showed increased binding in “tau-rich” regions in AD brains, and a significant overlap in areas stained with anti-tau antibodies.Reference Zhang, Arteaga and Cashion 24 Further, [18F]T807 and [18F]T808 tracers showed fast brain uptake followed by a rapid washout in normal mice, suggesting low nonspecific binding.Reference Xia, Arteaga and Chen 23 , Reference Zhang, Arteaga and Cashion 24 However, [18F]T807 was insensitive to differences between APPswe-Tau and wild-type background mice.Reference Fodero-Tavoletti, Okamura and Furumoto 15
The first [18F]T807 PET imaging results obtained in healthy controls (HCs), mild cognitive impairment (MCI), and AD subjects showed favourable kinetics—with both rapid brain delivery and WM clearance—as well as low nonspecific WM and cortical binding in HCs. In patients with MCI and AD, a distinct pattern of tracer uptake was observed, relative to the cerebellum—comprising lateral temporal, mesial temporal, parietal, occipital, and frontal cortices—mirroring the current understanding of tau deposition, as described by Braak and Braak.Reference Chien, Bahri and Szardenings 32 Similar findings were obtained using [18F]T808, with increasing signal intensity within these regions noted as a function of clinical severity. Although difficult to determine because of the small sample size, these results suggest that a 30- to 50-minute imaging time point may prove sufficient with respect to differentiating HCs from subjects with AD, with a time frame of 80 to 100 minutes optimal for detection of smaller amounts of tau deposits.Reference Chien, Szardenings and Bahri 37
Benzothiazole Derivative: [ 11 C]PBB3
Following a screening of several fluorescent chemicals with affinity for β-sheet conformations, a PBB class of ligands was recently developed for visualization of diverse structural forms of tau inclusions. The most promising of these tracers is [11C]PBB3, which shows good pharmacokinetic properties and robust binding to tau inclusions in small animals and humans.Reference Maruyama, Shimada and Suhara 25
In vitro and ex vivo fluorescence microscopy showed that PBB3 clearly identified tau inclusions in tau transgenic mice (PS19 line, harbouring P301S FTDP-17 mutation). Additionally, in vitro autoradiography studies showed that [11C]PBB3 produced high-contrast signals in the tissue of PS19 mice and AD patients with low nonspecific binding. Ex vivo autoradiography likewise demonstrated selectivity for tau inclusions in the PS19 mouse model.Reference Maruyama, Shimada and Suhara 25 These findings were supported by preclinical in vivo assessment showing that [11C]PBB3 identified tau inclusions in the PS19 mouse model.
The first exploratory clinical [11C]PBB3 PET study for patients with probable AD showed minimal nonspecific WM binding and rapid brain delivery. [11C]PBB3 signal was found to accumulate in the limbic system in mild AD, with expansion to most cortical areas with progression through moderate AD, consistent with Braak stage V/VI. Interestingly, a slight retention of [11C]PBB3 was noted around the hippocampus in a normal control subject who had shown a decline of several points on the Mini-Mental Status Examination (MMSE), similar to early Braak stages. Finally, elevated [11C]PPB3 binding was noted in the basal ganglia of a patient diagnosed with corticobasal syndrome, supporting the use of [11C]PPB3 to detect tau lesions in both AD and non-AD tauopathies.Reference Maruyama, Shimada and Suhara 25
Summary of General Properties of Tau Radiopharmaceuticals
The association between autoradiographic and immunohistochemical findings across multiple brain regions in both human and transgenic mouse brain tissue revealed the colocalization of all tracers with immunoreactive tau antibodies, indicating high selectivity for tau aggregates. Furthermore, autoradiography studies have provided specific and nonspecific binding, number of available binding sites, and affinity, parameters crucial for the interpretation of PET studies (for review, see (Reference Laruelle, Slifstein and Huang39); Table 2). Additionally, the tau tracers here reviewed possess desirable properties for consideration as potential neuroimaging probes, including high lipophilicity, low molecular weight, high selectivity/affinity, and rapid plasma clearance.Reference Laruelle, Slifstein and Huang 39 , Reference Pike 40
Possibilities and Challenges for in vivo Tracking of Tau Pathology
Clinical PET using such tau radiopharmaceuticals should be viewed as a work in progress. Initial PET studies using [18F]THK523 ruled out its use in clinical settings because of elevated WM binding. However, optimized second-generation compounds remain under investigation and carry promise for future use because of their expected specificity for AD forms of tau. In the case of [18F]T807 and [18F]T808, clinical studies conducted thus far indicate their ability to track cognitive decline—as indexed by MMSE scores—as well as the propagation of tau pathology, according to Braak staging. In the case of [11C]PBB3, despite findings highlighting its potential to detect tau pathology in both AD and non-AD tauopathies, its short half-life (20 minutes) limits its use to imaging centers possessing an onsite cyclotron and specialized radiochemistry, making it unsuitable for widespread clinical use.
Based on these early clinical studies, tau tracers may facilitate the differential diagnosis of AD—where tau tangles are composed of equimolar 3R and 4R isoformsReference Kouri, Whitwell, Josephs, Rademakers and Dickson 41 —and non-AD tauopathies, where tau tangles contain predominantly 3R or 4R isoforms. When combined with complementary structural and functional imaging approaches, the use of tau tracers may allow for improved detection and characterization of mixed pathology and refine our current understanding of the link between tau deposition, metabolic change, and brain atrophy. At the same time, tau tracers may provide further insight into the link between alterations in tau protein distribution that accompany normal aging and performance decrements on neuropsychological measures in nondemented elderly individuals.Reference Mukaetova-Ladinska, Harrington, Roth and Wischik 42 In the context of clinical trials involving tau-directed therapeutics, tau PET ligands may serve as diagnostic biomarkers to guide population enrichment strategies, to calculate sample size, and to increase the statistical power via population stratification or through use as baseline predictors.Reference Wu, Rosa-Neto and Gauthier 43 In parallel, tau-ligands can serve as endpoint biomarkers to monitor the rate of disease progression as well as response to therapy. Finally, tau ligands may aid in the testing of new hypotheses, including the hypothesis that β-amyloid and tau pathology arise independently in sporadic AD, with incident β-amyloid pathophysiology interacting synergistically with an antecedent limbic/brainstem tauopathy.Reference Jack, Knopman and Jagust 10 , Reference Braak and Del Tredici 44
Despite the promise held by the tracers discussed here, a number of challenges remain. Given that structural polymorphisms are the rule rather than the exception,Reference Villemagne 45 with a given tau isoform assuming various conformations, and different isoforms assuming similar forms ultrastructurally,Reference Wegmann, Jung, Chinnathambi, Mandelkow, Mandelkow and Muller 46 the distribution of such tau aggregates within the brain varies by phenotype.Reference Villemagne 45 Though an advantage in terms of differential diagnosis, the underlying assumption—namely, that binding of tau ligands will be comparable across the spectrum of tau polymorphisms—is unlikely.Reference Villemagne 45 In addition, the ability of tracers to bind tau may be affected by different posttranslational modificationsReference Martin, Latypova and Terro 47 such that a given radiotracer may be able to bind PHF-tau but not other ultrastructural tau conformations, such as straight filaments or randomly coiled filaments. Additionally, tau deposition from normal aging must be addressed to provide a reliable threshold and avoid false positives given the presence of tau pathology in a certain percentage of cognitively normal individuals.Reference Braak and Braak 48 , Reference Knopman, Parisi and Salviati 49 Finally, additional PET studies incorporating increased subject numbers and a wider range of tauopathies are necessary to further validate the use of tau ligands in clinical settings.
Conclusion
The growing interest in tau radiopharmaceutical will surely contribute to significant advancements in the field. Indeed, it is expected that many additional tau-focused radiopharmaceuticals will soon be examined. Current tau tracers hold translational value in terms of characterizing the link between the progression of tau pathology and cognitive impairment as well as in terms of monitoring treatment effects in clinical trials using tau-focused therapeutics. Finally, tau tracers hold the potential for inclusion as biomarkers of neurodegeneration, to be used alone or in parallel with CSF tau measurements in order to achieve an improved understanding of tauopathies.
Acknowledgements and Funding
The authors thank Professors Victor Villemagne and Noboyuki Okamura for providing Figure 1.
This work was supported by the Canadian Institutes of Health Research (CIHR; MOP-11-51-31), Alzheimer’s Association (NIRG-12-259245), Fonds de Recherche du Québec - Santé (FRQS; Chercheur Boursier), the Allan Tiffin Trust (Infrastructure), the Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq, Brazil), Fundação de Amparo à Pesquisa do Rio Grande do Sul (Fapergs, Brazil), and INCT for Excitotoxicity and Neuroprotection/CNPq.
Statement of Authorship
ERZ and AL contributed equally to this work. ERZ and AL were responsible for the conception and design of the review and for drafting and revising the manuscript. SG and PRN were responsible for revising the manuscript.






