Highlights
-
Door-in-door-out (DIDO) metrics for patients transferred for endovascular thrombectomy are relatively long in Québec (median 102 minutes), similar to other North American jurisdictions.
-
DIDO metrics are not associated with 90-day functional outcomes or mortality among patients treated by endovascular thrombectomy following transfer from a primary stroke center to a comprehensive stroke center.
-
Patient presentation outside of working hours is associated with DIDO ≥ 60 minutes.
Introduction
Acute ischemic stroke is a time-dependent medical emergency. Intravenous thrombolysis and endovascular thrombectomy (EVT) constitute the current reperfusion armamentarium for ischemic stroke, but their benefits on functional outcomes are time dependent. As such, optimal patient care relies on the rapid and organized succession of stroke identification and emergency medical service activation in the prehospital setting, followed by evaluation and treatment in an appropriate stroke center. Reference Boulanger, Lindsay and Gubitz1 While intravenous thrombolysis is readily accessible in numerous primary stroke centers (PSC), EVT is typically available in a more limited number of hospitals due to the centralization of neurointerventionalist expertise and specialized technical platforms available only at comprehensive stroke centers (CSC). Since timely EVT delivery remains a challenge in most healthcare systems, there is a pressing need to identify strategies to optimize the acute stroke care workflow.
The door-in-door-out time (DIDO) refers to the time spent in the PSC emergency department prior to transfer to the CSC for EVT. This potentially compressible window may constitute a period of undue delay in acute ischemic stroke management and therefore represent an opportunity for patient flow optimization to improve clinical outcomes. While the Canadian Cardiovascular Society and the American College of Cardiology/American Heart Association both recommend aiming for a DIDO ≤ 30 minutes in patients with ST-elevation myocardial infarction initially evaluated in a center without percutaneous coronary intervention services, Reference Wong, Welsford and Ainsworth2 there are no recent formal guideline-recommended DIDO targets for acute ischemic stroke, with those published in 2013 prior to widespread implementation of EVT suggesting target delays ≤ 120 minutes. Reference Alberts, Wechsler and Jensen3,4 In 2018, Canadian Stroke Best Practices set a DIDO of less than 45 minutes as a key quality indicator for acute stroke care, but this target was not supported by robust evidence. 5
Québec is the second most populous Canadian province (9 million residents) and covers a vast area of 1,667,441 km2 (approximately four times that of Germany). About 8000 individuals are hospitalized annually for acute stroke in the province, and approximately half of acute ischemic stroke patients who are treated with EVT in the province are initially evaluated at a PSC emergency department prior to being transferred to a CSC for the procedure. Reference Azzi, Boothroyd, Lambert, Nicolae and de Guise6 EVT is currently available at five CSCs across four major population hubs in the province Montreal: Centre hospitalier de l’Université de Montréal and Centre universitaire de santé McGill; Quebec City: Centre hospitalier universitaire de Québec; Sherbrooke: Centre hospitalier de l’Université de Sherbrooke; Saguenay: Hôpital régional de Chicoutimi; (Figure 1). Prehospital care systems in the province have implemented PSC bypass protocols to minimize delays to EVT for patients meeting specific criteria during paramedic evaluation. For instance, in the greater Montréal regions, individuals suspected of acute stroke are immediately transported to one of two CSCs in Montréal when their Cincinnati Prehospital Stroke Scale score is 3/3. Individuals with a score of 2/3 or less are directed to the closest PSC. Reference Nehme, Deschaintre and Labrie7 Hospital prenotification for suspected acute stroke typically occurs when patients are directly transported to a CSC but is not uniformly implemented for transport to a PSC.
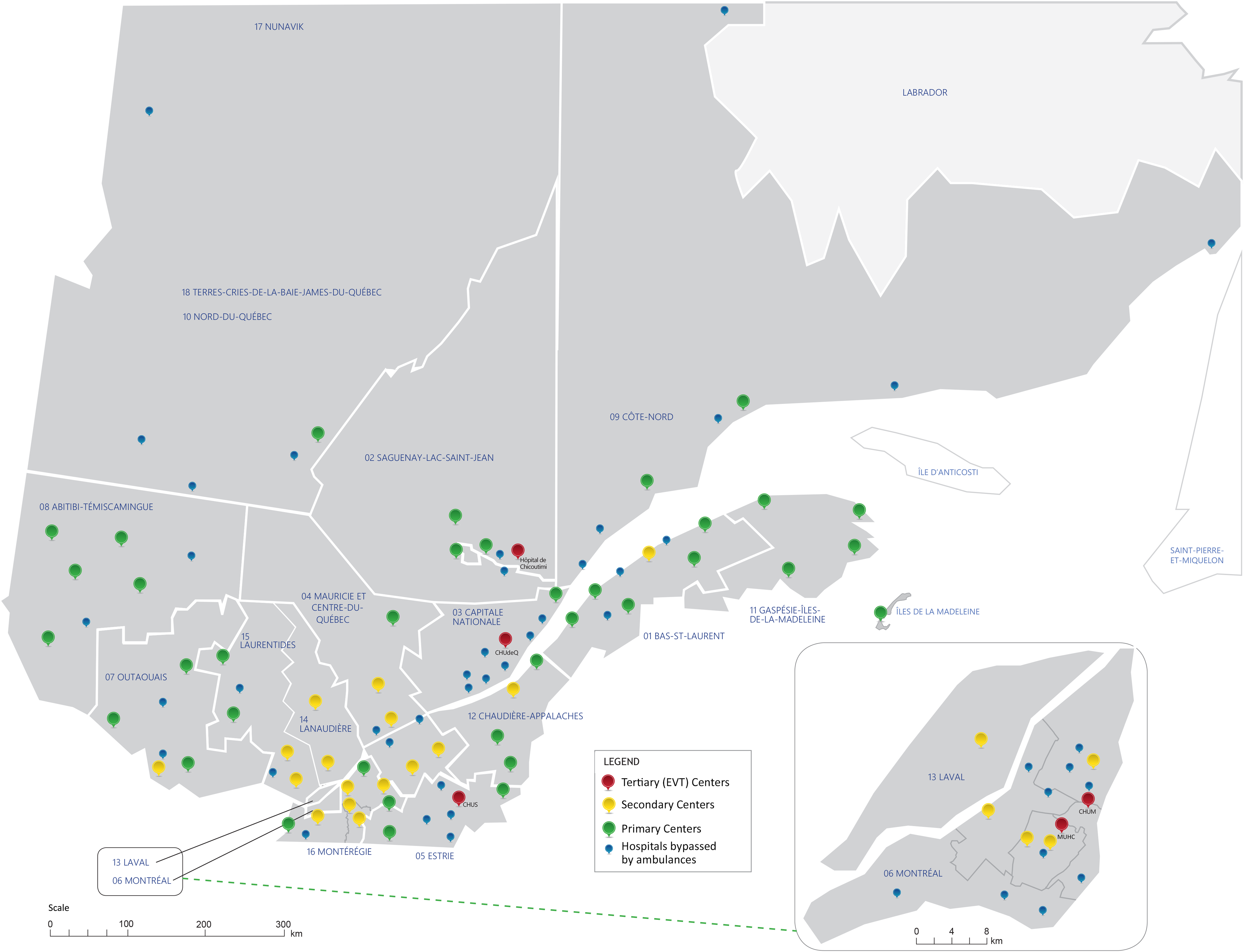
Figure 1. Location of hospitals in the stroke care network of Québec, Canada. Figure adapted with permission from INESSS. Reference Azzi, Boothroyd, Lambert, Boivin and Verteuil30 AIS = acute ischemic stroke; EVT = endovascular thrombectomy; CHUM = Centre hospitalier de l’Université de Montréal; MUHC = Centre universitaire de santé McGill (McGill University Health Center); CHUdeQ = Centre hospitalier universitaire de Québec; CHUS = Centre hospitalier universitaire de Sherbrooke; INESSS = Institut national d’excellence en santé et services sociaux du. Québec.
Considering the paucity of data on DIDO metrics for acute ischemic stroke and their potential impact on functional outcomes, our primary objective was to study the association between DIDO and 90-day functional outcomes in the province of Québec, Canada. Our secondary objective was to investigate the association between patient clinical and workflow characteristics and DIDO.
Methods
Study reporting follows guidance from the STROBE statement. Reference von Elm, Altman, Egger, Pocock, Gøtzsche and Vandenbroucke8 This study was approved by the Institutional Review Board of the Centre hospitalier de l’Université de Montréal (#MP-02-2022-10361). As a retrospective provincial registry-based study, no individual patient consent was required. However, each participating CSC provided institutional approval for the use of patient data from their respective institutions. All aggregate data pertinent to the study are presented in the manuscript and its Supplemental Material.
Study design
This study was a retrospective cohort study for which the target population comprised adult (≥18 years of age) individuals with an acute ischemic stroke who were first evaluated at a PSC and eligible for EVT as per standard selection criteria during the study period. Reference Boulanger, Lindsay and Gubitz1 We included consecutive acute ischemic stroke adult patients transferred for EVT by ground ambulance from a PSC to one of the five CSCs in the province of Québec, Canada, between April 2017 and March 2020. We excluded patients who were directly evaluated for acute stroke in a CSC emergency department, patients already hospitalized at the time of stroke onset (in-hospital strokes) and patients transferred by air, since helicopter transportation is not available in the province of Québec and plane transfer is rare.
Variables and measurement
For all included patients, we collected baseline characteristics (age, sex, pre-stroke functional status based on the modified Rankin scale [mRS]), stroke characteristics and management at the PSC (onset time, time of first contact with healthcare services, prehospital notification, arrival time at PSC, National Institutes of Health Stroke Severity Scale [NIHSS] score at PSC emergency department evaluation, acute ischemic changes on non-contrast brain CT as per the Alberta Stroke Program Early CT Score [ASPECTS], location of arterial occlusion on CT angiography [internal carotid artery, M1 and M2 segments of the middle cerebral artery], thrombolysis administration, departure time from PSC emergency department), transportation time between PSC and CSC, management at the CSC (arrival time at CSC, arterial puncture time, modified treatment in cerebral infarction [mTICI] score) and vital status and functional outcome at 90 days, as measured by the mRS. We calculated DIDO as the time spent in the PSC emergency department prior to departure for the CSC. Our co-primary outcomes were a favorable functional outcome at 90 days (mRS 0–2) and death at 90 days (mRS = 6).
The Institut national d’excellence en santé et services sociaux du Québec (INESSS; https://www.inesss.qc.ca/) collected data and metrics on all acute ischemic stroke patients treated by EVT in Québec between 2017 and 2020 as part of its governmental mandate to describe the state of acute stroke care in the province and recommend areas for improvement for policymakers. Reference Azzi, Boothroyd, Lambert, Nicolae and de Guise6,Reference Azzi, Boothroyd, Lambert, Nicolae and Guise9 INESSS, whose mission is to promote clinical excellence and the efficient use of resources in the health and social services sector to better support decision-making and improve practices, was responsible of tracking the number of hospital admissions of adults in Québec with a main diagnosis of stroke according to their region of residence, in the period from April 1, 2017, to March 31, 2020, in the provincial MED-ÉCHO databank. Clinical data collection for adults who had presented in an emergency department with a diagnosis of acute ischemic stroke and who were treated with EVT was carried out by medical archivists from INESSS, in collaboration with clinical teams at the five EVT centers. INESSS professional medical archivists are specialized in stroke and cardiovascular disease and use a common manual of procedures under the supervision of local and central medical teams comprised of stroke nurses and physicians. They obtained clinical information directly by manual review of each patient’s in-hospital medical chart at all five CSCs and entered data into a standardized case report form. Trained physicians, research nurses or research coordinators at each respective CSC obtained vital and functional status at 90 days by means of an in-person or telephone assessment with each respective patient or their proxy. For this study, CSC identities were masked and labeled as A, B, C, D and E following data dissemination agreements with the data provider.
Statistical analyses
We provide descriptive statistics for the entire cohort, reporting continuous variables as means and standard deviations or medians with first and third quartiles, as appropriate, and dichotomous variables as counts and proportions. We compared DIDO between the referral territories of the five CSCs using one-way analysis of variance (ANOVA).
In primary analyses, we assessed the associations between DIDO and co-primary outcomes (favorable functional outcome and death at 90 days post-stroke onset), with their corresponding 95% confidence intervals, using logistic mixed effects models with intercept random effects for the CSC, modeling DIDO in 1-minute increments and adjusting for relevant potentially confounding covariables identified using a directed acyclic graph (age, sex, pre-stroke mRS, NIHSS, ASPECTS and thrombolysis administration). Patients with unknown DIDO and with missing 90-day data (losses to follow-up) were excluded from primary analyses. We also performed subgroup analyses by recanalization status after EVT (mTICI 0–2a vs mTICI 2b3). As sensitivity analyses, we first modeled DIDO in 5-, 10-, 15- and 30-minute increments. We then considered only patients with reasonable symptom-onset-to-puncture delays (binary with an a priori cut-off of 270 minutes based on clinically reasonable maximum delay). We also assessed the association between DIDO and mRS at 90 days using an ordinal logistic regression model, adjusting for the same covariates (“shift analysis”).
In secondary analyses, we assessed the unadjusted associations between patient characteristics and DIDO ≥ 60 minutes, with their corresponding 95% confidence intervals, using logistic models. We chose a dichotomous outcome (DIDO < 60 vs ≥ 60 minutes) a priori as it reflects an achievable clinical target based upon published literature. Reference Choi, Tsoi and Pope10 Patient characteristics were determined a priori based on clinical plausibility: age, sex, prehospital prenotification, arrival at PSC outside daytime hours (5 PM–8 AM), arrival at PSC during a weekend, pre-stroke mRS, NIHSS, CT angiography done at the PSC, ASPECTS, evaluation by an in-person neurologist at the PSC and thrombolysis administration at the PSC. For variables with an unadjusted analysis p-value < 0.10, we then assessed variables’ adjusted associations with DIDO ≥ 60 minutes with their corresponding 95% confidence intervals using logistic mixed effects models with intercept random effects for the CSC and adjusting for relevant potentially confounding covariables. Patients with unknown DIDO were excluded from secondary analyses.
We defined statistical significance based on a p-value < 0.05. All analyses were performed on RStudio version 2023.06.0 + 421 (Posit Software, PBC, Boston, MA). 11
Results
Among 1354 patients screened for enrollment, 841 were transferred from a PSC to a CSC for EVT, including 51 patients with futile transfers (EVT not performed after transfer) and 790 patients fulfilling study selection criteria (Figure 2). Baseline descriptive statistics for the cohort are detailed in Table 1. The mean age was 69 (14) years, 400 (51%) patients were female, the median pre-stroke mRS was 0 (0–1) and the median NIHSS was 16 (10–20). The median delay from stroke symptom onset to arrival at PSC was 56 (40–83) minutes. Emergency medical services prenotified the PSC of stroke suspicion in 248 (31%) of cases. Brain non-contrast CT and brain and neck CT angiography were completed at the PSC in 759 (96%) and 662 (84%) of patients, respectively. The median ASPECTS was 9 (8–10). CT angiography revealed occlusion of the internal carotid artery in 147 (19%), the M1 segment of the middle cerebral artery in 468 (59%) and the M2 segment of the middle cerebral artery in 145 (18%) patients. Among 472 (60%) patients evaluated by a neurologist at the PSC, 422 were assessed by a neurologist at the bedside, and 50 were assessed via neurology teleconsultation. Intravenous thrombolysis was administered to 423 (54%) patients prior to transfer to the CSC. The median transportation time between the PSC and the CSC was 40 (26–56) minutes.
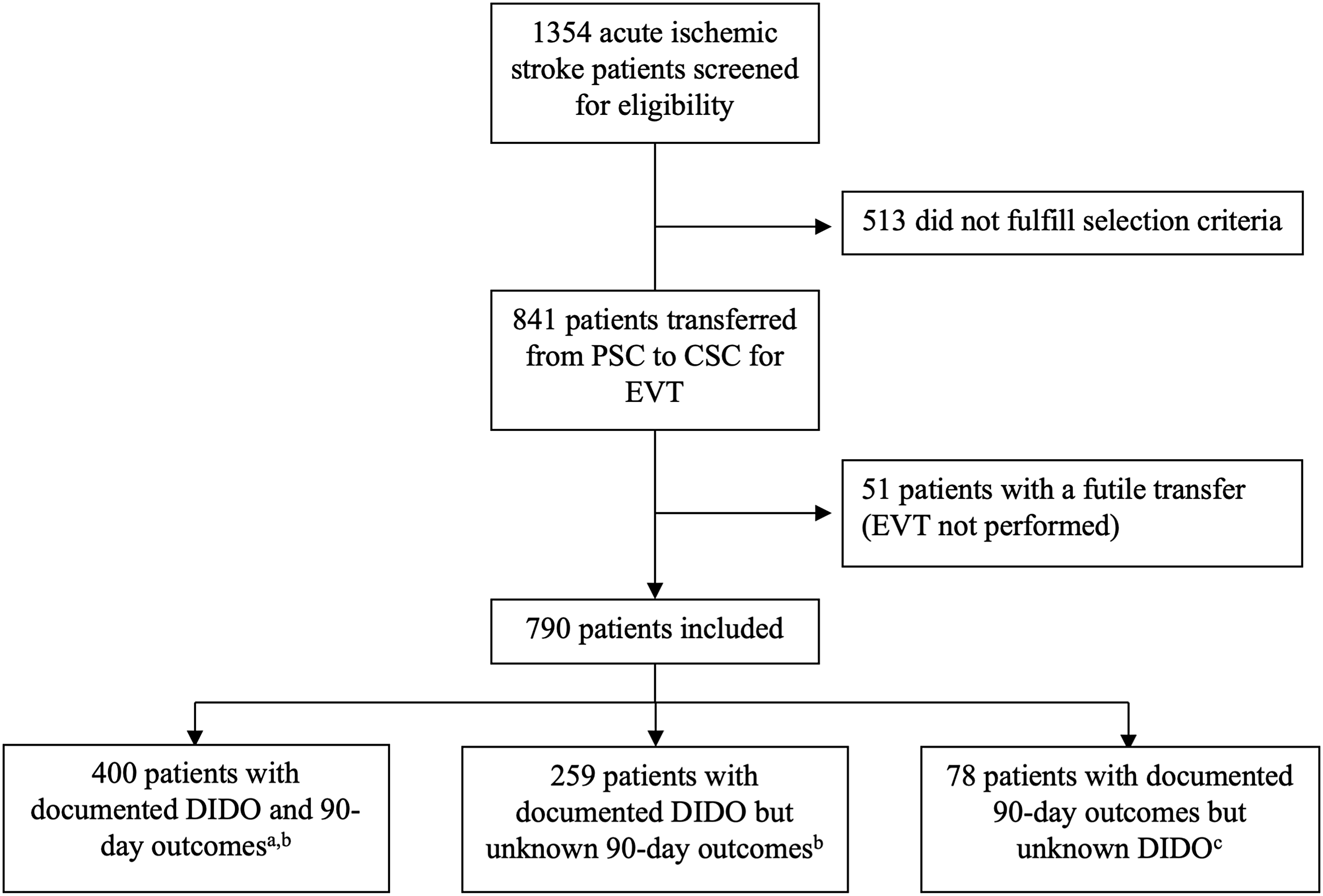
Figure 2. Patient flowchart. aIncluded in primary analyses. bIncluded in secondary analyses. cNot included in primary or secondary analyses. PSC = primary stroke center; CSC = comprehensive stroke center; EVT = endovascular thrombectomy; DIDO = door-in-door-out time.
Table 1. Descriptive statistics for the study cohort
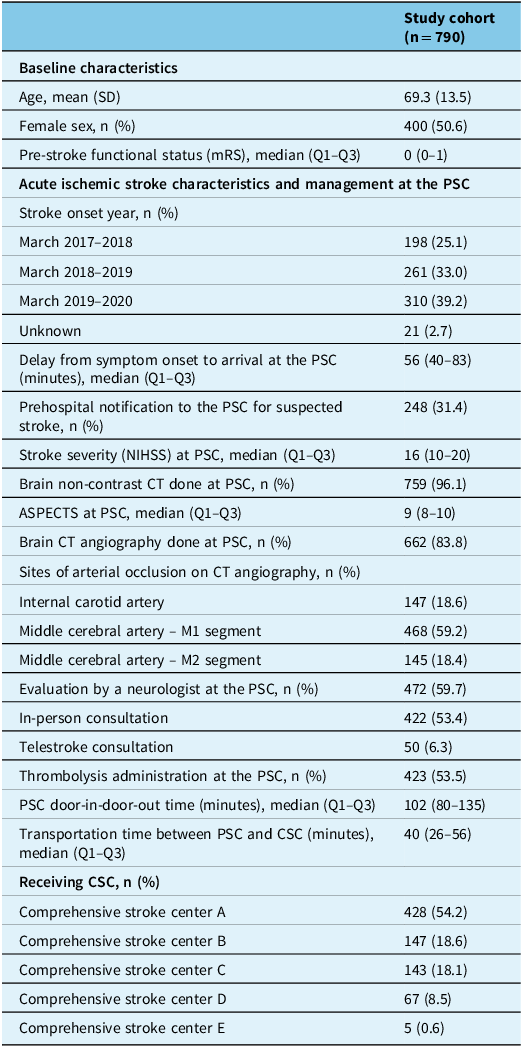
SD = standard deviation; IQR = interquartile range; PSC = primary stroke center; CSC = comprehensive stroke center; NIHSS = National Institutes of Health Stroke Severity; ASPECTS = Alberta Stroke Program Early CT Score.
During the study period, median DIDO was 102 (80–135) minutes (Figure 3A). Annual DIDO metrics were similar over the three years of observation. There was no significant difference in DIDO between the five CSCs (one-way ANOVA p = 0.10; Figure 3B).
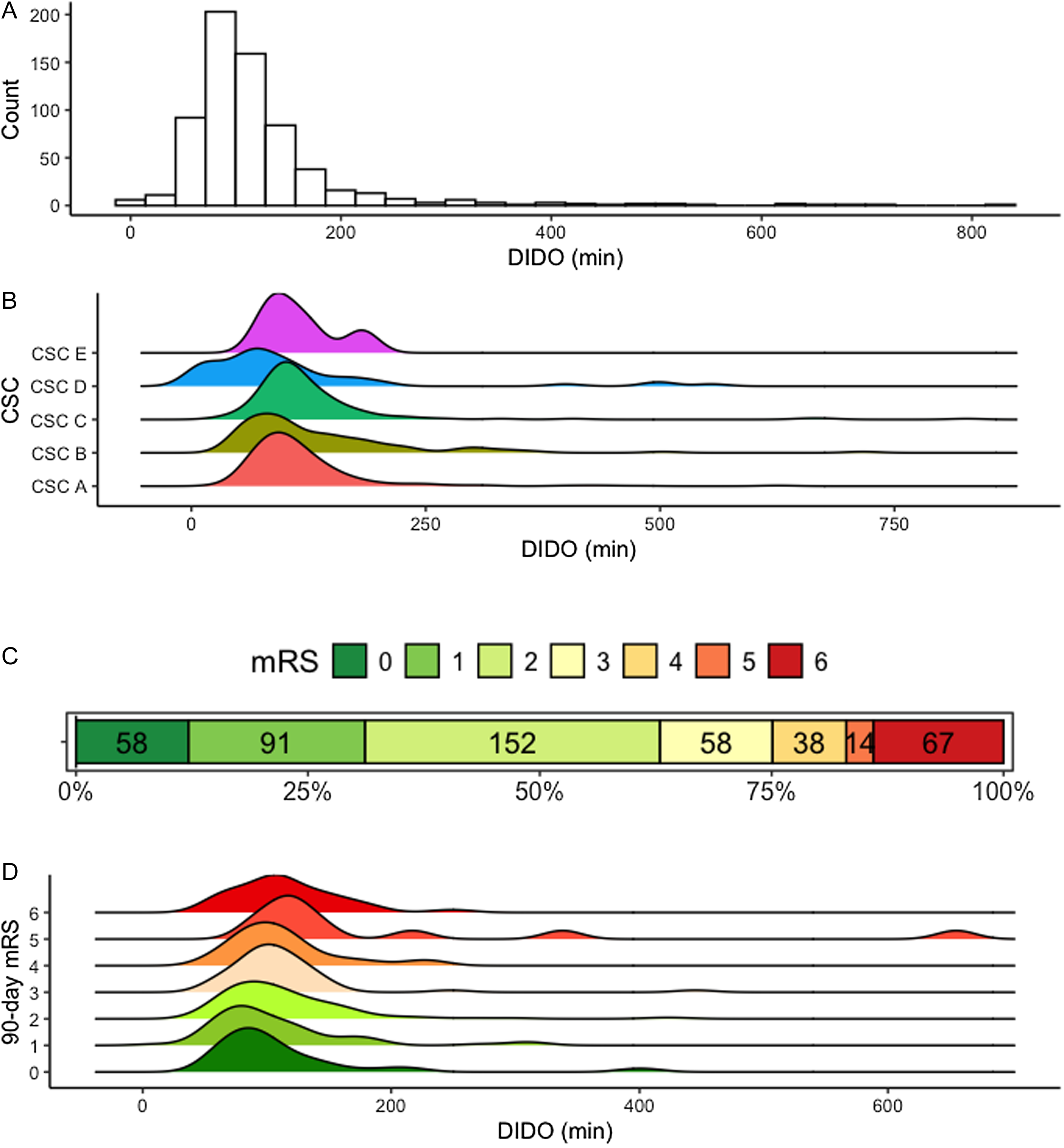
Figure 3. Door-in-door-out times and 90-day functional outcomes. Panels: (A) Histogram of door-in-door-out times for the study cohort. (B) Ridgeline plot of door-in-door-out times stratified by comprehensive stroke center. (C) Distribution of 90-day modified Rankin scores (with number of patients per score). (D) Ridgeline plot of door-in-door-out times stratified by 90-day functional modified Rankin score. DIDO = door-in-door-out time; mRS = modified Rankin scale score; CSC = comprehensive stroke center.
Data on management at the CSC are provided in Supplemental Table 1. The median CSC door-to-puncture was 24 (14–39) minutes. Favorable mTICI of 2b–3 occurred, respectively, in 660 (84%) and 542 (69%) patients.
Data were available on vital status and on functional status at 90 days for 701 (89%) and 478 (61%) patients, respectively (Supplemental Tables 2–3). Overall, 301 (63%) patients achieved a favorable functional outcome of mRS 0–2, and 67 (14%) died (Figure 3C).
DIDO was not associated with a favorable functional outcome (aOR for 1-minute increments: 1.00, 95% CI [0.99–1.00], p = 0.54) or death (aOR for 1-minute increments: 1.00, 95% CI [0.99–1.01], p = 0.69) at 90 days. Results were similar in the recanalization status subgroups (TICI 0–2a vs TICI 2b–3). In sensitivity analyses, point estimates were unchanged when modeling DIDO by increments of 5, 10, 15 or 30 minutes. Associations between DIDO and co-primary outcomes were also unchanged when considering only patients with a symptom-onset-to-puncture delay of <270 minutes. In ordinal logistic regression analysis, DIDO was not associated with an mRS shift at 90 days (aOR: 1.00, 95% CI [1.00–1.01], p = 0.87). In model diagnostics (not shown), we observed that the logit of the dependent variables (clinical outcomes) plotted against the independent variable (DIDO) yielded a relatively flat line, suggesting that there is no DIDO cut-off that is related to better outcomes.
Results from univariate and multivariate models for associations between patient characteristics and DIDO ≥ 60 minutes are provided in Table 2. In univariate models, arrival at the PSC outside daytime hours (i.e., between 5 PM and 8 AM; OR: 3.21, 95% CI [1.73–6.33], p < 0.01), pre-stroke mRS (OR: 1.46, 95% CI [0.99–2.34], p = 0.07), CT angiography done at the PSC (OR: 4.02, 95% CI [2.21–7.18], p < 0.01) and neurology evaluation at the PSC (OR: 1.84, 95% CI [1.05–3.21], p = 0.03) were marginally associated (p < 0.10) with DIDO ≥ 60 minutes. Among these variables, only arrival at the PSC outside daytime hours (aOR: 3.28, 95% CI [1.26–8.51], p = 0.01) was significantly associated with DIDO ≥ 60 minutes in multivariate analyses.
Table 2. Regression model results for patient characteristics versus door-in-door-out times ≥ 60 minutes
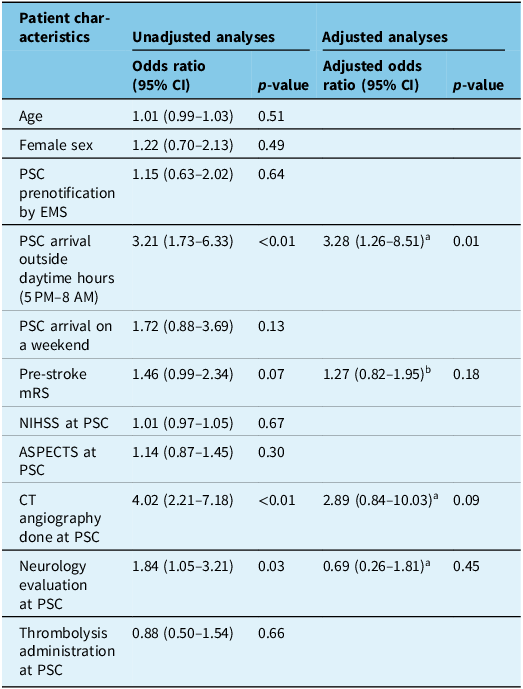
CI = confidence interval; PSC = primary stroke center; EMS = emergency medical services; mRS = modified Rankin scale; NIHSS = National Institutes of Health Stroke Severity; ASPECTS = Alberta Stroke Program Early CT Score. aModel covariates: age, sex, premorbid mRS, NIHSS and delay between symptom onset and arrival at PSC. bModel covariates: age and sex.
Discussion
In this large retrospective cohort study of acute ischemic stroke patients treated with EVT between 2017 and 2020 in the province of Québec, Canada, we did not find significant associations between DIDO and either favorable functional outcomes or death at 90 days. Among patient characteristics, PSC presentation outside of daytime hours (between 5 PM and 8 AM) was associated with DIDO ≥ 60 minutes. Overall, we documented DIDO that were relatively long, stable over time and not significantly different between the province’s five CSC territories.
Previous studies on the association between DIDO and functional outcomes in acute ischemic stroke have shown mixed results. Although few, recent studies have suggested that DIDO may be associated with functional outcomes in acute ischemic stroke Reference McTaggart, Moldovan and Oliver12,Reference Saver, Goyal and van der Lugt13 and also amenable to improvement. Reference Choi, Tsoi and Pope10,Reference Sablot, Farouil, Laverdure, Arquizan and Bonafe14,Reference Kollikowski, Amaya, Stoll, Müllges, Schuhmann and Pham15 However, other studies demonstrated no significant associations with mRS at 90 days, Reference Choi, Tsoi and Pope10,Reference Saver, Goyal and van der Lugt13 and one study concluded that shorter DIDO did not lead to faster initiation of EVT. Reference Kollikowski, Amaya, Stoll, Müllges, Schuhmann and Pham15 These conflicting findings may indicate that 90-day mRS is not sufficiently sensitive to document the marginal effect of DIDO on acute ischemic stroke outcomes. Reference Ospel, Ganesh and Goyal16 It is also possible that the effect of DIDO on functional outcomes might not be linear, for instance, in patients with collateral failure, whereby further decreases in DIDO do not lead to changes in outcomes. Alternatively, DIDO might not have an effect on outcomes in patients selected for EVT, so long as they still meet criteria for recanalization at the time they arrive at the CSC. The effect of DIDO on 90-day mRS may also be modified by the effect of EVT: in one study, DIDO were found to be associated with 90-day mRS only among patients with failed recanalization, which may suggest that the robust benefit of successful recanalization is not significantly modified by delays attributable to longer DIDO. Reference McTaggart, Moldovan and Oliver12 However, in our study, the lack of association between DIDO and outcome was not modified by recanalization status.
Our findings on DIDO metrics are similar to those from prior studies conducted in other regions of North America (mean or median DIDO between 85 and 207 minutes) and in Europe (66–141 minutes). Reference Sablot, Farouil, Laverdure, Arquizan and Bonafe14,Reference Kollikowski, Amaya, Stoll, Müllges, Schuhmann and Pham15,Reference Ng, Low and Andrew17–Reference Flores, Seró and Gomez-Choco20 In 2013, prior to the widespread adoption of EVT as an urgent acute ischemic stroke therapy, the Brain Attack Coalition and the Joint Commission recommended a DIDO ≤ 120 minutes. Reference Alberts, Wechsler and Jensen3,4 Some have argued this target lacks universal consensus and objectivity, Reference Hsu, Yang and Hsu21 and many prior studies have shown that this target is infrequently achieved in practice. Reference Stamm, Royan, Giurcanu, Messe, Jauch and Prabhakaran22–Reference Kuc, Isenberg and Kraus24 In 2018, Canadian Stroke Best Practices set a DIDO of <45 minutes as a key quality indicator for acute stroke care, but this target was not robustly justified by the current literature. 5 Expert guidance based on the best available scientific evidence is therefore required to determine appropriate DIDO targets.
Studies documenting variability in DIDO within other large territories are sparse. Similarly to our study, DIDO in Rhode Island were relatively homogeneous. Reference McTaggart, Moldovan and Oliver12 However, studies conducted in Australia have found that DIDO were heterogeneous across PSC, Reference Ng, Low and Andrew17 with significant differences between centers in metropolitan and regional settings. The geographic and demographic determinants of DIDO within and across territories have not been thoroughly investigated.
In our cohort, the only factor documented to be significantly associated with longer DIDO was arrival at the PSC outside daytime hours (5 PM–8 AM), where additional delays for stroke workup may occur. Prior studies have suggested that female sex, Black race or Hispanic ethnicity, Reference Stamm, Royan, Giurcanu, Messe, Jauch and Prabhakaran22 initial misdiagnosis of acute ischemic stroke, Reference Prabhakaran, Khorzad and Parnianpour25 medical conditions such as atrial fibrillation and heart failure, stroke-specific characteristics such as basilar occlusion or need for vascular imaging Reference Kuc, Isenberg and Kraus24 and center-dependent factors such as regional PSC location as opposed to metropolitan Reference Wong, Dewey and Campbell26 or treatment at a non-certified stroke center Reference Kuc, Isenberg and Kraus24 were associated with longer DIDO. Failure to detect severe strokes and large vessel occlusions on arrival at the PSC has been reported to be the most common failure leading to longer DIDO. Reference Holl, Khorzad and Zobel27 Therefore, immediate vascular imaging at PSCs, while marginally prolonging evaluation times, is critical to identifying patients with large vessel occlusions amenable to EVT. Conversely, factors associated with faster DIDO in prior literature include EMS prenotification, higher NIHSS score, IV thrombolysis, CT angiography at the PSC and use of the same ambulance crew for interhospital transport. Reference Ng, Low and Andrew17,Reference Stamm, Royan, Giurcanu, Messe, Jauch and Prabhakaran22,Reference Kuc, Isenberg and Kraus24,Reference Victor, Bian and Mamdouh28 The challenge remains to determine and implement the optimal management strategy in terms of patient flow across the entire continuum of care leading to EVT: for instance, delays at the PSC must be minimized, but sufficient to permit accurate identification of acute ischemic stroke eligible for EVT and administration of IV thrombolysis.
Studies have suggested strategies to shorten DIDO and have documented improvements in delays with the implementation of quality improvement processes. Reference Saver, Goyal and van der Lugt13,Reference Victor, Bian and Mamdouh28,Reference Azzi, Boothroyd, Lambert, Boivin and Verteuil30 A continuous quality improvement program implemented over 4 years yielded a median DIDO of <60 min. Reference Choi, Tsoi and Pope10 In a single-center high-volume CSC observational study, execution of a simple protocol for the rapid identification of acute ischemic stroke cases evaluated at a PSC and requiring transfer to a CSC for EVT led to a decrease in median DIDO from 104 to 64 minutes. The protocol consisted of three key implementations: CSC notification on patient arrival to the PSC, CT and CT angiography imaging performed concurrently within 30 min of PSC arrival and PSC imaging data shared with the CSC through a cloud-based platform. Patients evaluated using the full protocol were twice as likely to have a favorable outcome (50% vs 25%), but this analysis was not adjusted for potential confounders. Reference McTaggart, Yaghi and Cutting29
Notable strengths of our study include the sample’s large size, covering an entire healthcare system, the spectrum of patients reflecting clinical practice and the robust data measurement methodology based on source documents and expert medical archivists. Our study also has several limitations. First, the study design did not capture patients who did not receive EVT despite potential eligibility, as well as patients transferred to a CSC for EVT who did not ultimately receive the therapy (“futile transfers”). This may limit the generalizability of our findings and potentially underestimate the impact of DIDO on patient outcomes, notably by excluding patients who may not have received EVT due to overly long management delays at the PSC. Second, functional outcomes at 90 days were assessed by personnel unblinded to clinical information. This is unlikely to have induced an information bias since DIDO was not readily calculated or available in the clinical charts used by assessors of vital and functional status. Third, missing data on DIDO and/or 90-day functional status was common in our database. However, participants with DIDO and outcome data were overall comparable to those with missing data on measured variables (Table S3), although the impact of missing data on results was not formally assessed. Although we did not adjust for certain variables that are likely associated with 90-day functional outcomes, such as collateral status, time from symptom onset to PSC arrival, time from symptom onset to EVT, quality of in-hospital stroke unit care and access to rehabilitation, these are unlikely to be independently associated with DIDO and are therefore not likely confounders in our association of interest. Finally, since we dichotomized our functional status outcomes, we may have missed more subtle associations between DIDO metrics and patient outcomes. However, ordinal analysis yielded similar results, and categorical outcomes like favorable functional status and death are likely the most meaningful for patients, their families and policymakers.
Based on our findings, further research is required to understand the determinants of DIDO in PSC, to develop strategies aimed at minimizing DIDO through quality improvement and to estimate the effect of DIDO on patient outcomes. Importantly, future research should clarify the impact of DIDO on functional status in the wider population of patients who present to a PSC with symptoms of acute ischemic stroke, including those who do not benefit from EVT due to delays in stroke identification and workup, poor collateral status or complications from intravenous thrombolysis. This work could lead to the identification of evidence-based PSC time targets for completing initial acute stroke workup and initiating CSC transfer that would optimize patient outcomes.
In conclusion, DIDO for acute ischemic stroke in the Canadian province of Québec are relatively long, similar to other North American jurisdictions. In this sample of acute ischemic stroke patients treated by EVT following transfer from a PSC to a CSC, DIDO was not associated with 90-day functional outcomes or mortality, though patient presentation outside of working hours was associated with DIDO ≥ 60 minutes. Future research should focus on studying the impact of DIDO on functional status in the wider population of patients who present to a PSC with symptoms of acute ischemic stroke, on identifying the most robust determinants of DIDO across different populations and on implementing strategies to minimize delays while optimizing acute ischemic stroke and large vessel occlusion diagnosis, particularly outside daytime hours, to enhance the timeliness and benefit of EVT.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/cjn.2025.70.
Acknowledgments
The authors thank INESSS personnel involved in data acquisition and organization as well as all PSCs and CSCs that contributed data to the provincial database and the current study.
Author contributions
JNB and AYP designed the study. LA and MLH managed data extraction from the provincial INESSS database. JNB conducted data analysis. AS and JNB drafted the manuscript. All other authors revised the manuscript for intellectual content. All authors reviewed and approved the final version of the manuscript. AYP is the guarantor of the study.
Funding statement
BR is supported by the Centre for Clinical Brain Sciences of the University of Edinburgh (Rowling and Dr Hugh S P Binnie scholarship) and the Canadian Institutes of Health Research (CIHR; Doctoral Foreign Study Award, DFD-187711).
Competing interests
The authors declare no competing interests.







