Introduction
Parkinson’s disease (PD) is an age-related movement disorder characterized by resting tremor, rigidity, bradykinesia, and postural instability. Its prevalence is dramatically increased after age 60. The etiology of PD is believed to be multifactorial resulting from both genetic and environmental factors, with less than 10% having a causative gene mutation identified.Reference Lill1, Reference Abdullah, Basak, Patil, Alves, Larsen and Møller2
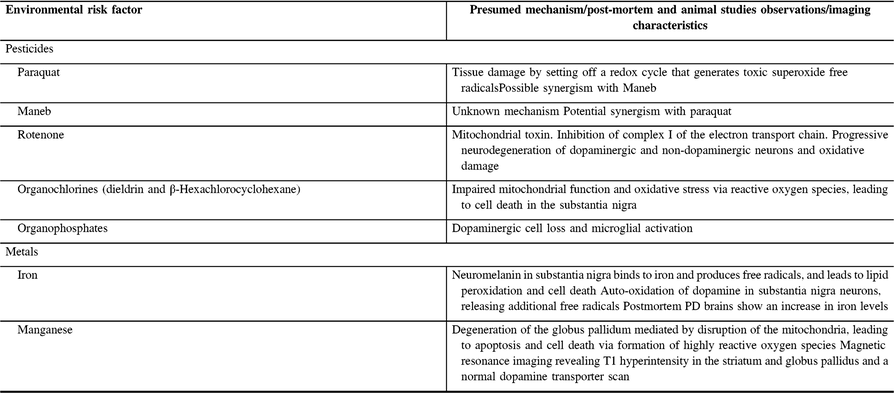
Based on epidemiology and toxicology studies, an important line of research focuses on the role of pesticide exposure associated with the risk of PD.Reference Ballard, Tetrud and Langston3–Reference Sánchez-Santed, Colomina and Herrero Hernández6 Some pesticide families including herbicides such as paraquat, fungicides, insecticides, or rodenticides containing organochlorides and organophosphates can cause serious damage to the nervous system. They contribute to the development of PD by including oxidative stress, mitochondrial dysfunction, α-synuclein fibrillization, and neuronal cell loss.Reference Nandipati and Litvan5, Reference Sánchez-Santed, Colomina and Herrero Hernández6 In addition, exposure to metals such as iron and manganese (through welding, battery manufacturing, long-term parenteral nutrition, and IV synthetic drug use) has also been associated with an increased risk for PD.Reference Bharath, Hsu, Kaur, Rajagopalan and Andersen7–Reference Gorell, Johnson and Rybicki10 Postulated mechanisms of action of toxic substances on the pathogenesis of PD are summarized in Table 1 (based on recent reviews from Nandipati and LitvanReference Nandipati and Litvan5 and Sánchez-Santed et al.Reference Sánchez-Santed, Colomina and Herrero Hernández6).
Table 1: Postulated mechanisms of action of toxic substances associated with an increased risk of PDReference Nandipati and Litvan5, Reference Sánchez-Santed, Colomina and Herrero Hernández6
Other factors reflecting both genetic and environmental influences on PD phenotype are vascular risk factors (VRFs) and sex. Vascular leukoencephalopathy and VRFs, which are thought to be mediated by genetic predispositions and lifestyle variables, predispose to a more severe PD symptomatology and more prominent cognitive features.Reference Malek, Lawton and Swallow11, Reference Pilotto, Turrone and Liepelt-Scarfone12 Women were also found to have a delayed onset compared to men (by about 2 yearsReference Haaxma, Bloem and Borm13, Reference Twelves, Perkins, Uk and Counsell14), a difference that could be partly explained by differences in intracerebral estrogen levels, leading to higher striatal dopamine levels. This difference in estrogen levels can be explained by an intrinsic lower postsynaptic dopamine D2 receptor affinity in womenReference Pohjalainen, Rinne, Någren, Syvälahti and Hietala15 and by exogenous factors such as past pregnancies, gynecological surgeries, and hormonotherapy.Reference Ragonese, D’Amelio and Salemi16
Although specific genes have been associated with earlier onset forms of PD,Reference Lill1 the data available regarding the influence of environmental factors on age-at-onset (AAO) are scarce. Earlier, AAO was observed in 15 former weldersReference Racette, McGee-Minnich, Moerlein, Mink, Videen and Perlmutter17 and in 188 patients with prior exposure to hydrocarbon.Reference Pezzoli, Canesi and Antonini18 Similar results were obtained in 36 patients exposed to pesticides and/or heavy metals.Reference Ratner, Farb, Ozer, Feldman and Durso19 One study focusing on AAO in relation to multiple environmental risk factors was retrieved.Reference Maher, Golbe and Lazzarini20 The authors studied 203 sibling pairs with PD and found only one environmental factor that influenced AAO, that is, a history of head trauma. However, the authors point out that their results need to be interpreted cautiously since the high genetic contribution to PD in this sample may have overshadowed potential environmental influences.
The aim of the present study is to confirm the hypothesized relation between environmental risk factors (pesticides and toxic metals exposure through welding) and AAO of PD, in a larger cohort, defined with a clinic-based ascertainment scheme.
Methods
Participants
We used an incident cohort of 290 patients recruited through three designated movement disorder clinics in the province of Quebec, Canada. Every index case was evaluated by a neurologist and met the Ward and GibbReference Ward and Gibb21 criteria for idiopathic PD. Additionally, patients needed to be dopa-responsive and to have been diagnosed no more than 10 years previously. Informed written consent form was obtained for each participant, and the study was approved by the ethics committee of the recruitment centers.
Data Collection
Participants and their partners completed a detailed questionnaire regarding social, professional, and medical history. The questionnaires included sociodemographic data (age, sex, and education), health outcomes at enrolment, and other conditions likely to impact on health like smoking. Patients were asked about their occupational history, especially those at risk for exposures to pesticides. It comprised specific questions regarding any past job in the following fields: manufacturing, farming, forestry, golf or green space maintenance, automobile, chemical products and pesticides spraying, agriculture products, and welding.Reference Maher, Golbe and Lazzarini20
For each of these past jobs, information on dates of beginning and end of each activity was collected. A positive occupational history was considered when PD patients reported to have experienced at least 6 months of metals and/or pesticides exposition. Patients were asked if they had been exposed to (or directly manipulated) the following: herbicides, fungicides, insecticides, rodenticides, or other unknown substances of similar nature. They rated the frequency and duration of exposures on a 4-point ordinal scale: less than 1/month for <10 years; less than 1/month for >than 10 years; more than 1/month for <10 years; and more than 1/month for >10 years.
Statistical Analysis
The studied cohort consisted of 290 patients from the Quebec City area in eastern Canada. The final analysis included 256 patients whose status regarding professional exposures was detailed. Among these, 76 reported professional exposures to pesticides, toxic metals or both; 20 confirmed directly manipulating pesticides; 30 confirmed being directly exposed to toxic metals through welding; and 4 of them both manipulated pesticides and were former welders.
The dependent variable was age at diagnosis, which is considered to be the most standardized estimate of AAO, free from recall bias and determined by a neurologist. The main independent variable was prior professional exposure to toxic substances. It was further subdivided into nature of the exposure (pesticides and welding), frequency, and proximity. Analysis was conducted with SPSS 24.0 software (SPSS Inc., Chicago, IL, USA).
Primary Analysis
The primary analysis focused on two groups of patients: patients without professional exposures (Control, N = 170) and those exposed to pesticides or welding (All exposures, N = 76). The mean age at diagnosis in both groups was compared with an analysis of covariance (ANCOVA), adjusting for potential confounders that were sufficiently documented in our sample: sex, number of VRFs, place of residence (antecedent of living near a farm or vegetable garden – within 1 km for more than 6 months), which increases the risk of indirect exposure to pesticides, and smoking. Significant covariates would be subsequentially included in secondary group comparisons.
Secondary Analysis
In secondary analyses, the All exposures group was further subdivided into two subgroups (Pesticides, N = 53 and Welding, N = 30). Both groups were treated independently with separate ANCOVAs, since data regarding pesticides exposure or welding were incomplete in some cases and would have reduced the sample size in each group in a combined analysis; moreover, only four patients had antecedents of both welding and exposure to pesticides, rendering a factorial plan futile.
For the Pesticides group, two “exposure–response” models were tested with multiple linear regression analyses, the first with regards to the proximity of exposures (no contact, N = 170; at-risk-job, N = 33; and direct manipulation, N = 20) and, the second, the frequency of exposures (≤1 per month, N = 12 and >1 per month, N = 38), for a minimal duration of six consecutive months were associated to AAO. The frequency of exposure was chosen over the duration given the potential confounding effect of age on duration, with older patients having longer overall working experience. Significant covariates identified in the ANCOVA would be included in the regression models.
Frequency and proximity analyses were not performed in former welders, since they all had high frequency and proximity exposure to toxic metals.
A α-value of 0.05 was used as the signification threshold for all tests.
Results
Sample Characteristics
The descriptive statistics for the cohort and the subgroups are presented in Table 2. The mean age at diagnosis was 58.0 years (SD = 0.69). The sample included 167 men (65%) and 89 women (35%).
Table 2: Clinical and demographic features of patient’s cohort
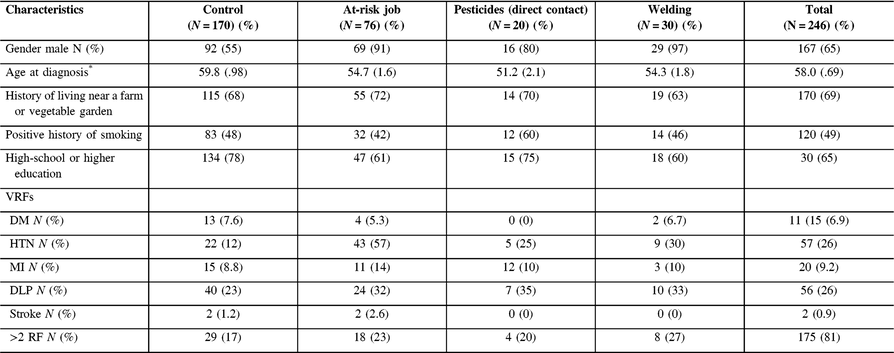
DM=diabetes mellitus; HTN=hypertension; MI=myocardial infarction; DLP=dyslipidemia; RF=risk factor.
* Mean (standard deviation).
Exposure to Pesticides and Welding Is Associated with Earlier Onset of PD
Homogeneity of the variances among the different cohort subgroups was confirmed with Levene’s tests, allowing for the use of parametric tests. Figure 1 presents the mean AAO in the different groups and subgroups. The main ANCOVA included 42 exposed patients and 81 controls in which information about all variables and covariates was sufficient. It revealed a significantly earlier AAO, F(1, 117) = 9.25, p = 0.003, in exposed patients. The number of VRF was found to be the only significant covariate (p = 0.0005; the more VRFs, the latter the AAO).
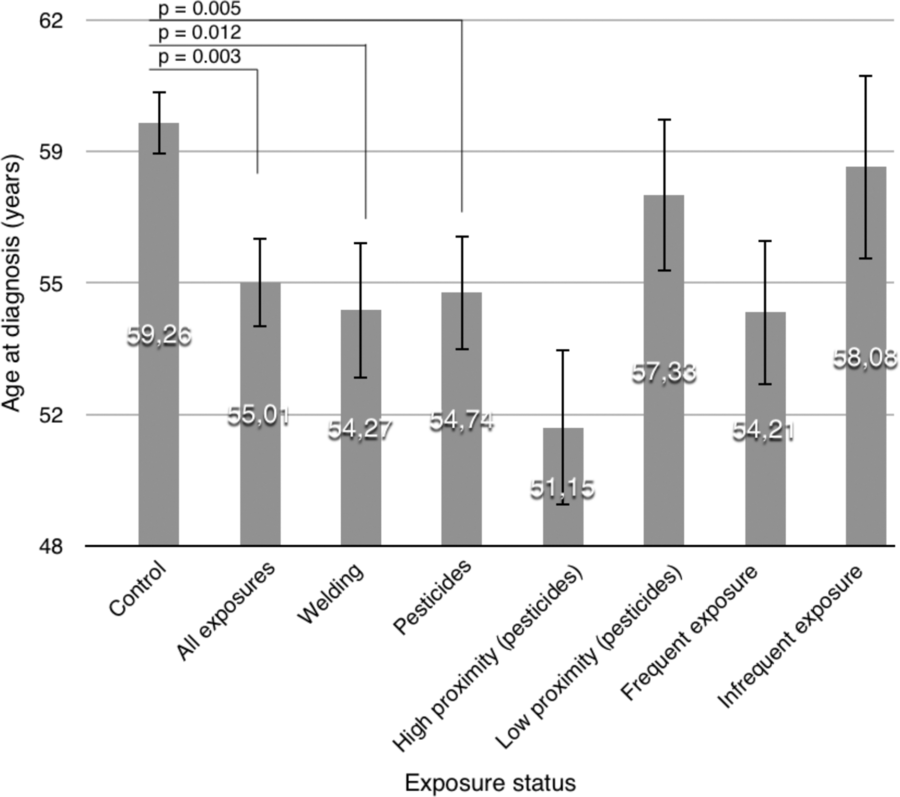
Figure 1: Age at diagnosis as a function of exposure status. Error bars represent the standard error of the mean. p-values are indicated for all mean comparisons subjected to formal inferential testing.
Subgroup ANCOVAs using the number of VRF as a covariate showed that both pesticides (N = 65, F(1, 231) = 5.76, p = 0.017) and welding (N = 30, F(1, 198) = 17.66, p = 0.012) were associated with earlier onset compared to the control group.
Proximity of Exposures to Pesticides
The mean AAO according to proximity groups is depicted in Figure 1. The linear regression analysis shows that a model including proximity and the number of VRFs significantly predicts AAO (R 2 = 0.093, p = 0.00003). Proximity is a significant, negatively correlated, independent predictor (B s = −0.138, p = 0.035), and the number of VRFs is significantly positively correlated to AAO (B s = 0.284, p = 0.00002).
Frequency of Exposures to Pesticides
The mean AAO according to frequency groups is depicted in Figure 1. The linear regression model including frequency and the number of VRFs also significantly predicts AAO (R 2 = 0.333, p = 0.00003). Proximity is a significant, negatively correlated, independent predictor (B s = −0.207, p = 0.001), and the number of VRFs remains significantly positively correlated to AAO (B s = 0.266, p = 0.00004).
Discussion
Although there is a clear association in the literature between exposures to pesticides or heavy metals and the risk of PD,Reference Bellou, Belbasis, Tzoulaki, Evangelou and Ioannidis22 how such exposures affect the course of the disease remains unclear. This article reports convincing evidence that occupational exposure to toxic substances influences the AAO of PD, supporting prior evidence of such an association. Exposure to pesticides and welding were both associated with an earlier onset, an effect that was greater with higher levels of exposure, both in terms of frequency and proximity.
Such findings support current – and encourage the development of further – models in which AAO is determined not only by genetic predispositions, but also by exogenous neuronal insults over an extended period of time.Reference Abdullah, Basak, Patil, Alves, Larsen and Møller2 This represents an important step forward into determining the variables that lead to neuronal death as the brain ages. Environmental exposures have been associated with PD through several mechanisms leading to cellular dysfunction and eventually neuronal death.Reference Nandipati and Litvan5, Reference Sánchez-Santed, Colomina and Herrero Hernández6 How these insults combine with genetic factors probably represents the key to understanding the AAO of PD.
Epidemiological studies are pivotal to identify the biological targets warranting scientists’ attention in order to develop prevention strategies and disease modifying therapies in PD.Reference Ravina, Fagan and Hart23 Our ability to target high-risk individuals is desirable since they are the most likely to benefit from eventual therapeutic optionsReference Ascherio and Schwarzschild24 and knowing that some of these are at risk of developing the disease earlier might contribute to optimize the timing of interventions.
Validity of the Study
The main findings of this study are consistent with prior reports in the literature, notably with the results of Ratner et al.Reference Ratner, Farb, Ozer, Feldman and Durso19 who used a data collection method similar to ours. The addition of an “exposure–response” profile and controlling for many variables reported to be associated to PD risk increases the robustness of the observed effects.
One strength of this study resides in the clinic-based ascertainment scheme, since PD requires diagnostic expertize to differentiate it from other types of movement disorders (e.g. essential tremor, Lewy Body disease, and cerebrovascular disease). This approach minimized the risk of contaminating the cohort with misdiagnosed patients.
Using a case-only design also takes away several potential sources of bias, notably recall and sensitivity bias. However, such designs are also prone to other forms of biases. Wilk and LashReference Wilk and Lash25 outlined that differences in AAO may reflect generational trends in the prevalence of exposure to the risk (or protective) factors. One finding that could be attributable to such a bias in this study is the positive relationship between AAO and VRF. Indeed, since VRFs are associated with a clinically more severe PD syndrome,Reference Malek, Lawton and Swallow11, Reference Pilotto, Turrone and Liepelt-Scarfone12 one could have hypothesized that it would lead to an earlier onset, which is contrary to the results obtained in the present study. The simple – and probable – explanation for this seemingly paradoxical result is that older patients from our cohort, as is the case in the general population, have more accumulated VRFs acquired in an age-dependent manner.Reference Song, Mitnitski and Rockwood26 As regards the effect of occupational exposures on AAO, if such a bias was in play, it would have led to its underestimation. Indeed, exposure to occupational factors should be intrinsically lower in young patients (who have less working experience), which might falsely associate lower exposure levels to earlier AAO. The opposite inclination in our results strengthens their validity. Looking at the frequency of exposures instead of their duration also reduced the potential impact of such a bias.
Limitations
The main limitation of the current study is the relatively small sample size and the missing data in some subgroups, which prevented the use of a full-factorial plan. Multiple regression analyses were used in order to underline an “exposure–response” profile according to the frequency and proximity of exposures. Traditionally, such analyses are performed on much larger sample sizes, although some authors found that a very small numbers of subjects per variable allows adequate estimation of regression coefficients, standard errors, and confidence intervals.Reference Austin and Steyerberg27 The small number of women in the exposure groups did not allow to consider stratified analysis to assess if the observed effects are applicable to both men and women. Although previous findings showed no significant difference in mean age of onset of PD among male and female,Reference Ratner, Farb, Ozer, Feldman and Durso19, Reference Maher, Golbe and Lazzarini20 we could not fully exclude the possibility of a small contribution of sex differences to the main effect.
All potential confounders could not be controlled for since the data were either not included in the questionnaires or incomplete (e.g. physical activity, caffeine, specific medication, …). The missing data in some subgroups limited our ability to test separately the association between exposure to specific pesticides and the AAO of PD. The lack of genetic data that may influence AAO also limits the appreciation of a potential interaction with the studied environmental factors. Moreover, the questionnaire items regarding frequency and proximity to exposure are rather vague, which possibly leads to heterogeneous exposure levels within the different subgroups.
Conclusion
Although this study reinforces the notion that environmental exposures affect the AAO of PD, epidemiological studies on larger cohorts are still warranted in order to better identify, among people with prior exposures, who is at risk of developing PD, and who will do so earlier. Such studies should notably be aimed at identifying which specific pesticides compounds are particularly noxious and determining if their effect depends on individuals’ genetic profiles.
Acknowledgements
We are deeply grateful to all patients for their participation in this study. We thank Dr. Myers Richard for kindly providing the questionnaire. Access to the data for this research has been made possible through the Réseau Parkinson du Québec, Canada.
Disclosures
Dr. ZG-O reports personal fees from Sanofi Genzyme, personal fees from Lysosomal Therapeutics Inc., personal fees from Idorsia, personal fees from Prevail Therapeutics, personal fees from Denali, and personal fees from Inception Sciences, outside the submitted work. The other authors have no conflicts of interest to declare.
Statement of authorship
1. Research project: Conception: P-LG, ND; Organization: P-LG, ND, ZG-O; Execution: P-LG.
2. Statistical analysis: Design and execution: P-LG; Review and critique: ND and ZG-O.
3. Manuscript preparation: First draft: P-LG; review and critique: All authors.





