Traumatic brain injury (TBI) is the leading cause of death and disability in people younger than age 45 in the United States. 1 Falls and motor vehicle accidents are the leading causes of TBI.Reference Faul, Xu, Wald and Coronado 2 According to the Centers for Disease Control and Prevention, 1.7 million TBIs occur annually in the United States and TBI is associated with 30.5% of all injury-related deaths. In 2010, the economic burden of TBI in the United States, which includes direct medical and indirect costs such as loss of productivity, was estimated to be $76.5 billion.Reference Finkelstein, Corso and MIller 3
The Glasgow Coma Scale (GCS) is the most widely used clinical scoring system in the assessment of TBI severity. Persons with a GCS score of 3 through 8 are classified as having a severe TBI, 9 through 12 as moderate TBI, and 13 through 15 as mild TBI. Although mild TBIs or concussions represent 75% of annual TBIs in the United States, the costs related to severe TBI hospitalizations account for approximately 90% of the TBI-related cost. However, mild concussions (including those not seen in an inpatient hospital setting) can also be associated with significant ongoing costs in terms of disability, lost work, or neuropsychiatric complications.Reference Thurman 4 , Reference DeKosky, Blennow, Ikonomovic and Gandy 5 In addition, 43% of patients that require hospitalization continue to have cognitive, social, or physical deficits 1 year postinjury.Reference Finkelstein, Corso and MIller 3 , Reference Selassie, Zaloshnja, Langlois, Miller, Jones and Steiner 6
Thus the immediate and long-term consequences of all ranges of TBI create significant economic and societal burden. Understanding the epidemiology of TBI is essential to shape public health policy, implement prevention strategies, and justify allocation of resources toward research, education, and rehabilitation in TBI. Current scientific literature contains wide variations in the incidence of TBI. Although there have been reviews on the epidemiology of TBI, most are not systematic,Reference Bruns and Hauser 7 , Reference Corrigan, Selassie and Orman 8 focus only on one TBI type (e.g. mild TBI),Reference Cassidy, Carroll, Peloso, Borg, von Holst and Holm 9 or are very specific to one population (e.g. homeless people,Reference Topolovec-Vranic, Ennis, Colantonio, Cusimano, Hwang and Kontos 10 incarcerated individualsReference Farrer and Hedges 11 ).
Furthermore, a recent meta-analysis of 15 studies on the prevalence of TBI in the adult general populationReference DeKosky, Blennow, Ikonomovic and Gandy 5 reported that 12% of adults in developed countries had a history of TBI. Definitions of TBI remain imprecise in the literature, and this persisting problem has led to wide epidemiological estimates that vary according to study design and data source. There has not been, to our knowledge, a systematic review of population-based studies addressing the international incidence of TBI that includes all subtypes. The goal of our study was to carry out a comprehensive and international systematic review and meta-analysis of the incidence of TBI and examine factors associated with estimate heterogeneity.
Methods
The systematic review and meta-analysis were conducted according to a predetermined protocol and established guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses).Reference Moher, Liberati, Tetzlaff, Altman and Group 12 The search strategy (Supplementary Appendix 1) was developed by study authors with expertise in TBI and/or epidemiology and in consultation with a research librarian with extensive systematic review expertise. The search was conducted on May 23, 2014, in the Medline and EMBASE databases using key words and topic headings related to TBI and concussion. References were exported and managed using EndNote X5. 13 All studies reporting data from 1985 onwards were eligible for inclusion in this review to identify studies after the introduction of magnetic resonance imaging in clinical practice. Articles were included if published in English or French; translation in other languages was not available for this study. Review articles on the epidemiology of TBI and the reference lists of included articles were also hand searched for additional articles.
Abstracts and titles of all references were screened independently, in duplicate, to identify original research that reported on the incidence of TBI. Abstracts that were clearly not population-based were excluded at this stage. Two reviewers independently screened the full-text articles of abstracts identified in the first phase. Articles were included if they met the following criteria: (1) original research, (2) population-based (sampling all members of a defined population or using probability-based sampling to select the population), and (3 reported an epidemiology estimate (e.g. prevalence, incidence, data needed to calculate an incidence estimate) of TBI. Prevalence papers were also identified to ensure an incidence estimate was not reported in it. Disagreements pertaining to the inclusion of articles were resolved by consensus and involvement of a third party as necessary.
Two reviewers extracted and reached agreement on data from included articles using a standard data collection form. When multiple articles reporting data from the same study population were encountered, the most comprehensive data was used. For example, numerous articles may report on data from the same registry. In cases where the articles reported on different data collection years or subgroups (sex, age), all data (every article) were included. Demographic data such as age, sex, and study location were recorded. Diagnostic data were collected, as were the sources of these data and definitions/diagnostic criteria for TBI. Incidence estimate(s) of TBI from each study were recorded, along with any stratification by age, gender, year of data collection, or TBI severity.
Incidence estimates were separated into two distinct groups: incidence proportion and incidence rate. The incidence rate of TBI is the number of new cases of TBI over the total amount of person-time at risk for having a TBI during a specified period. The incidence proportion is the number of new cases of TBI over the total number of people in the population at risk for having a TBI during a specified period. These distinct categories may account for different periods at risk and therefore were not combined in the current study.
Incidence estimates were stratified by the following variables: age, sex, continent, and TBI severity. Age was categorized as pediatric (<15 years of age), elderly (>65 years of age), or all ages. TBI severity was grouped into three mutually exclusive groups where available: mild, moderate, and severe. All analyses included persons of all TBI severities and ages (except when stratified based on these variables). Quality of the included studies was evaluated independently by two reviewers using an assessment tool (Supplementary Appendix 2) that was developed based on a previous study quality scoring system and published guidelines on assessing ascertainment bias in observational studies.Reference Boyle 14 , Reference Loney, Chambers, Bennett, Roberts and Strafford 15 The quality tool assessed sample representativeness, condition assessment, and statistical methods. Each study was given a quality score of 0 to 8 based on fulfillment of the quality criteria.
To assess for significant between-study heterogeneity, the Cochrane Q statistic was calculated and I 2 was used to quantify the magnitude of between-study heterogeneity. When statistically significant heterogeneity (Q statistic p value <0.05) was absent, the pooled estimate and 95% confidence intervals (CIs) were calculated using a fixed-effect model. When significant heterogeneity was present, a random-effects model was used. Publication bias was investigated visually using funnel plots and statistically using Begg’s and Egger’s tests.
For all tests, p<.05 was deemed to be significant. All statistical analyses were carried out in R, version 2.14 (R Development Core Team). The metafor package was used to produce the pooled estimates, forest plots, and publication bias assessment.Reference Schwarzer 16 - Reference Egger and Smith 18 The metafor package was used to conduct meta-regressions by age, sex, and so on using restricted maximum likelihood estimation.Reference Viechtbauer 19
Results
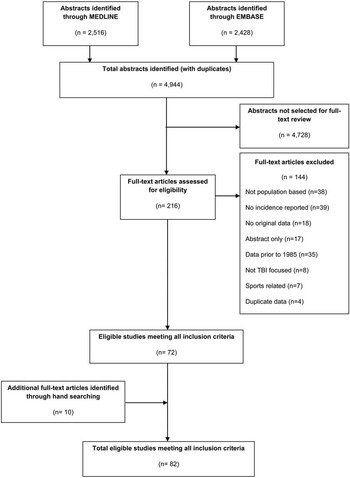
The results of the search strategy yielded a total of 4944 citations: 2,516 from Medline and 2,428 from EMBASE (Figure 1). After the initial screen, 216 articles met the criteria for full-text review, of which 144 were excluded (38 were not population-based, 39 did not report an incidence estimate of TBI, 18 were not original data, 17 were only abstracts, 13 reported data before 1985, eight did not focus on TBI, seven reported on sports-related injuries, and four reported on duplicate data sources). Hand searching resulted in the inclusion of ten additional articles. From the 82 eligible studies overall (original search and hand searching), 77 were included in the meta-analysis. Characteristics of the 82 included studies are shown in Supplementary Table 3A-D.
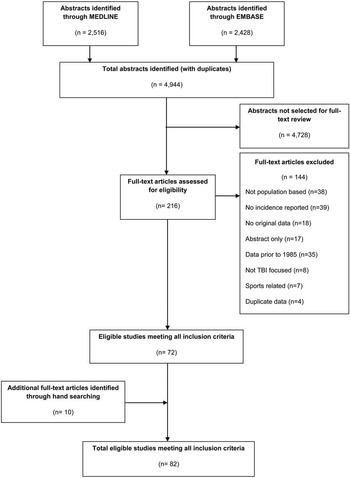
Figure 1 Flow chart of search strategy for traumatic brain injury (TBI). 4944 citations were originally identified. 216 full-text articles were assessed for eligibility. 10 studies were identified through hand searching; ultimately 82 studies were included in the systematic review.
All of the included studies reported on the incidence of TBI. Thirty-nine of the studies reported on data from North America, 32 from Europe, six from Australia, three from Asia, one from Africa, two from New Zealand, and one from South America (some studies reported data from more than one country).
Probability-based sampling was used in 18 studies, 20 - Reference Thurman, Alverson, Dunn, Guerrero and Sniezek 36 whereas the remainder of the included studies sampled the entire target population area. For example, Koepsell and colleaguesReference Koepsell, Rivara, Vavilala, Wang, Temkin and Jaffe 32 used a two-stage stratified sampling design to: (1) select hospital emergency departments and (2) select children with TBI presenting to the emergency room, though not admitted. In contrast, Engberg and colleaguesReference Engberg and Teasdale 37 used the Danish National Hospital register as their sampling frame, which covers more than 99% of all discharges in Denmark.
Incidence of Traumatic Brain Injury
Sixty-three studies 20 - Reference Jager, Weiss, Coben and Pepe 31 , Reference Sosin, Sniezek and Thurman 35 - Reference Coronado, McGuire, Sarmiento, Bell, Lionbarger and Jones 89 met all eligibility criteria for the incidence proportion of TBI, of which 39 met eligibility criteria for inclusion in the meta-analysis. Studies could only be included in the meta-analysis if they provided an incidence estimate with CIs or data required to calculate the CI. The pooled annual incidence proportion for pediatric (<15 years of age) populations using a random-effects model was 110 per 100,000 (95% CI: 34-358) (Figure 2). Significant heterogeneity existed between estimates, I 2 =100%, Q p value <0.0001. In elderly (>65 years of age) populations, the pooled annual incidence proportion was 166 per 100,000 (95% CI: 143-194). Significant heterogeneity existed between estimates, I 2 =100%, Q p value <0.0001. For those studies reporting on all age groups, the pooled annual incidence proportion was 295 (95% CI: 274-317).Significant heterogeneity existed between estimates, I 2 =100%, Q p value <0.0001. The estimates ranged from 69 per 100,000 in a North American study of all agesReference Langlois, Kegler, Butler, Gotsch, Johnson and Reichard 59 to 1750 per 100,000 in a New Zealand study of all ages.Reference McKinlay, Grace, Horwood, Fergusson, Ridder and MacFarlane 66
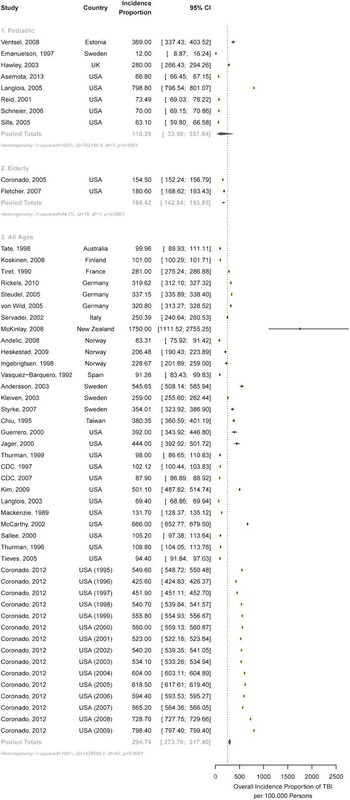
Figure 2 Incidence proportion of TBI, across age groups. Forest plot comparison of TBI incidence proportion by age subgroup. The horizontal bars depict corresponding 95% confidence intervals for each estimate. The incidence proportion of TBI in pediatric populations (110.26, CI: 33.98-357.84), elderly populations (166.42, CI: 142.84-193.89), and all ages (294.74, CI: 273.70-317.40) is shown.
Twenty studiesReference Bazarian, McClung, Shah, Cheng, Flesher and Kraus 24 , Reference Koepsell, Rivara, Vavilala, Wang, Temkin and Jaffe 32 , Reference Leibson, Brown, Ransom, Diehl, Perkins and Mandrekar 33 , Reference Sosin, Sniezek and Thurman 35 , Reference Alaranta, Koskinen, Leppanen and Palomaki 39 , Reference Arnarson and Halldorsson 41 , Reference Hillier, Hiller and Metzer 54 , Reference Nell and Brown 90 - Reference Walder, Haller, Bottequin, Schoettker, Ravussin and Brodmann 102 met all eligibility criteria for the incidence rate of TBI, and 14 were eligible for inclusion in the meta-analysis (Figure 3). In all age groups, the pooled annual incidence rate was 349 per 100,000 person-years (95% CI: 96-1266), with significant heterogeneity between studies, I 2 =100%, Q p value <0.0001. The pooled annual incidence rate in pediatric populations was 134 per 100,000 person-years (95% CI: 105-171), with significant heterogeneity between estimates, I 2 =100%, Q p value <0.0001. A random-effects model found the overall pooled annual incidence rate of TBI in adolescents, adults, and elderly to be 319 (95% CI: 313-326) per 100,000 person-years. No statistical heterogeneity existed between the estimates, I 2 =0%, Q p value 0.384. For those studies combining adolescents and adults, the pooled annual incidence rate was 618 per 100,000 person-years (95% CI: 603-634), with significant heterogeneity present, I 2 =94.3%, Q p value <0.0001.
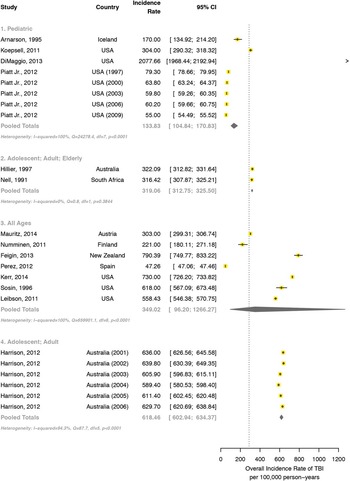
Figure 3 Incidence rate of TBI, across age groups. Forest plot comparison of TBI incidence rate by age subgroup. The horizontal bars depict corresponding 95% confidence intervals for each estimate. The incidence rate for combined adolescent and adult studies was significantly higher than that of pediatric populations and combined adult, adolescent, and elderly populations.
Sources of Heterogeneity
Age
For the incidence proportion of TBI, estimates were significantly higher in studies of all ages compared with elderly only (>65 years of age) studies. There were no significant differences between pediatric (<18 years of age) and elderly studies, nor pediatric and all ages studies.
Significant differences existed among all age groups in the incidence rate of TBI (Figure 3). The incidence rate for combined adolescent and adult studies was significantly higher than that of pediatric populations and combined adult, adolescent, and elderly populations. The incidence rate of TBI in pediatric studies was significantly lower than that of combined adolescent, adult, and elderly studies and that of combined adolescent and adult studies.
Sex
The incidence proportion of TBI in males was significantly greater than that of females. The pooled annual incidence proportion using a random effects model for females was 86 per 100,000 (95% CI: 71-105) and 151 per 100,000 (95% CI: 126-181) for males. Differences in incidence rate estimates of TBI between males and females did not reach statistical significance. The pooled annual incidence rate using a random-effects model for TBI in females was 195 per 100,000 person-years (95% CI: 84-452) and in males was 388 per 100,000 person-years (95% CI: 138-1092). Included studies did not provide sufficient information for us to be able to analyze sex differences stratified by age groups.
Continent
Meta-analysis by continent was available for both incidence proportion and rate of TBI (Figure 4) There was no significant difference in the pooled annual incidence proportion of TBI between European (228 per 100,000 [95% CI: 158-329]) and North American studies (331 per 100,000 [95% CI: 305-359]). One New Zealand study reported the highest incident proportion (1750 per 100,000 [95% CI: 1111-2755]); when combined with Australia, this gave the Australasian region an incidence proportion of 415 per 100,000 (95% CI: 25-6853). In incidence rate studies reporting on all age groups, there was no significant difference in the incidence rate of TBI between continents. The European rate was 147 per 100,000 person-years (pooled data, 95% CI: 33-649), the North American rate 632 per 100,000 person years (pooled data, 95% CI: 511-781), and the rate in one study from New Zealand 790 per 100,000 person-years (95% CI: 750-833).
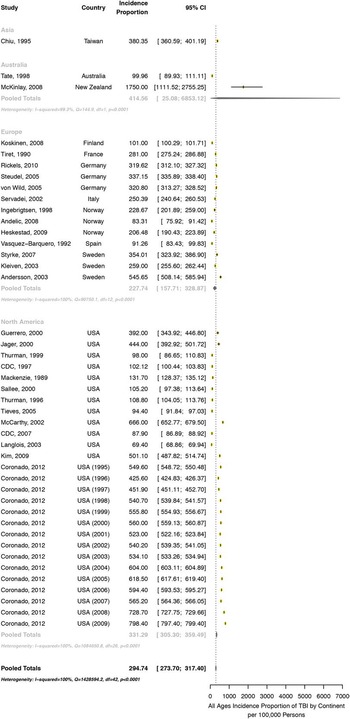
Figure 4 Incidence proportion of TBI, across continents. Forest plot comparison of TBI incidence proportion by continent subgroup. The horizontal bars depict corresponding 95% confidence intervals for each estimate. The incidence proportion of TBI was lower in Europe (227.74, CI: 157.71-328.87) than North America (331.29, CI: 305.30-359.49), Asia (380.35, CI: 360.59-401.19) or Australasia (414.56, CI: 25.08-6853.12).
Severity
Information on disease severity was available for both the incidence proportion and rate of TBI. There was a significant difference between all severity levels in the incidence proportion according to meta-regression analyses (p<0.05). The pooled annual incidence proportion of mild TBI was 224 per 100,000 (95% CI: 120-418), moderate TBI was 23 per 100,000 (95% CI: 18-29), and severe TBI was 13 per 100,000 (95% CI: 10-18).
There was also a significant difference between severity levels in the incidence rate of TBI according to meta-regression analyses (p<0.05). The pooled annual incidence rate of mild TBI was highest, at 982 per 100,000 person-years (95% CI: 756-1276), followed by moderate and severe TBI at 41 per 100,000 person-years, and severe TBI at 6 per 100,000 person-years (95% CI: 3-12).
Publication Bias
For the incidence proportion of TBI, significant funnel plot asymmetry was found for Begg’s (p=0.0037), but not Egger’s test (p>0.05). Upon visual inspection, the funnel plot appeared symmetrical. There was an insufficient sample size to calculate publication bias for the incidence rate and period prevalence of TBI.
Study Quality
The median study quality score for studies necessitating a response rate was 6/8 (range 4-7, n=69). For those not necessitating a response rate, the median study quality score was 6/6 (range 4-6, n=13). Eighty studies described the target population in detail and all sampled either the entire population or used probability sampling (Supplementary Table 4A,B). Sixty-four studies reported a response rate greater than 70%, and 65 articles adequately described the nonresponders. Almost all studies reported a sample that was representative of the target population (72/82). All studies used standardized data collection methods; however, only 17 reported using diagnostic criteria to assess for the presence of TBI. The majority of the studies (54) did not report estimates with their accompanying confidence intervals or by subgroups.
Discussion
TBI is an important cause of preventable morbidity and mortality worldwide. The economic and social impact of TBI is substantial because of both direct medical and rehabilitation costs and intangible costs resulting from disability and loss of productivity.Reference Zygun, Laupland, Hader, Kortbeek, Findlay and Doig 88 Many cases of TBI are either unrecognized by health care professionals or unreported by the patient, and TBI has thus been termed a “silent epidemic” as many cases of TBI are not captured in epidemiological statistics.Reference Rusnak 103 To implement effective preventive and treatment strategies for TBI, accurate information regarding the frequency of this condition in the general population is essential. We conducted a systematic review of the incidence of TBI with meta-analyses to estimate the incidence of this critical health problem globally and in important population subgroups.
There have been a number of literature reviews examining the incidence of TBI; however, these studies are not systematic,Reference Selassie, Zaloshnja, Langlois, Miller, Jones and Steiner 6 , Reference Bruns and Hauser 7 provide information on only one subtype of TBI patients (i.e. those with mildReference Cassidy, Carroll, Peloso, Borg, von Holst and Holm 9 or severe TBIReference Andelic, Anke, Skandsen, Sigurdardottir, Sandhaug and Ader 101 ), a specific population (i.e. age groupsReference Thompson, McCormick and Kagan 104 , incarcerated individuals), or focus on one geographic region.Reference Emanuelson and von Wendt 50 To the best of our knowledge, this is the first systematic review and meta-analysis that includes studies from around the world in all subgroups of TBI in all age groups. The results obtained provide valuable insight on the differences in TBI incidence worldwide, as well as factors that contribute to the heterogeneity of TBI incidence, which are important considerations when developing future studies examining TBI epidemiology.
In examining the method of data abstraction between the individual studies (Supplementary Table 3), diagnosis of TBI in a New Zealand studyReference McKinlay, Grace, Horwood, Fergusson, Ridder and MacFarlane 66 consisted of self-reported diagnosis via telephone interviews in addition to medical chart review, which may have resulted in a higher estimate that could be closer to the truth, although misclassification bias cannot be ruled out; conversely, a Finland studyReference Alaranta, Koskinen, Leppanen and Palomaki 39 obtained diagnoses via hospital administrative health databases and International Classification of Diseases (ICD) codes. As such, patients with minor TBI that may not present to hospital for treatment are not captured in the Finnish study.
The incidence rate of the pediatric population is 134 per 100,000 person-years, compared with a pooled annual incidence rate in all ages of 349 per 100,000 person-years. These numbers are significantly lower compared with the incidence rate of adolescent and adult populations (618 per 100, 000) in one Australian study.Reference Harrison, Berry and Jamieson 98 Pooled analyses of the incidence proportion of TBI show an estimate in the pediatric population of 110 per 100,000 persons, in the elderly of 166 per 100,000 persons, and an overall proportion estimate in all ages of 295 per 100,000 persons. From these estimates of incidence rate and proportion, it is reasonable to infer that age contributes to heterogeneity and the majority of TBI occur in the adult population compared with either pediatric or elderly populations.
Further sources of heterogeneity were identified in the subgroups of sex and severity, with a higher proportion of males sustaining head injury and the majority of TBI being classified as mild. These trends are likely attributable to the propensity for young adult males to be involved in risk-taking behavior,Reference Steinberg 105 with a resultant increased risk for head injury.
In looking at continental differences, pooled estimates were only available for incidence proportion of Europe and North America with single-study estimates for Asia, Australia, and New Zealand. The lowest incidence proportion was reported in Australia,Reference Tate, McDonald and Lulham 79 followed by Europe, North America, and Asia.Reference Chiu, Hung and Shih 46 The highest incidence proportion was reported from a study in New Zealand.Reference McKinlay, Grace, Horwood, Fergusson, Ridder and MacFarlane 66 All studies included in the analysis obtained diagnoses of TBI via hospital records except for the study from New Zealand,Reference McKinlay, Grace, Horwood, Fergusson, Ridder and MacFarlane 66 which included telephone surveys and self-reports as well as evidence of visits to a general practitioner with a primary complaint of head injury, therefore possibly capturing incidences of minor TBI that do not present to hospital. This undoubtedly contributes to the significantly higher incidence proportion estimates for that region. In studies that reported incidence rate across all age groups, the lowest incidence rate was reported in Europe, followed by North America and New Zealand. Several theories can be postulated from the continental differences in incidence rate and proportion of TBI. Public awareness, driven in part by professional sporting organizations such as the National Football League, has led to the development of legislation in 42 states and the District of Columbia surrounding TBI in sports.Reference Tomei, Doe, Prestigiacomo and Gandhi 106 , Reference Ellenbogen, Berger and Batjer 107 The resulting cognizance, education, and regulation have led to increased prevention and safety measures, thereby contributing to the lower incidence proportion observed in North American studies. Furthermore, improved traffic and safety regulations have contributed to a decline in motor vehicle accident–related TBI injuries in high-income countries such as Europe and North America.Reference Maas, Stocchetti and Bullock 108 Only single studies are available for incidence proportion and rate in Asia, Australia, and New Zealand. These studies may not represent the true incidence of head injury in this population; thus, additional epidemiological studies of head injury in these regions are necessary to draw reliable conclusions. A possible limitation of our study is that we did not include articles written in languages other than English or French; however, given the large number of studies we were able to include, we do not anticipate this to significantly alter the pooled annual incidence estimates.
The most significant limitation of our study lies in the considerable heterogeneity among studies. Several factors contribute to the heterogeneity in the analysis: (1) variations in methods of data collection and differences in identification of cases among studies; (2) underreporting of cases of TBI; and (3) inconsistencies in diagnostic definitions of TBI. Many studies included in our analysis identified cases of TBI through hospital and administrative datasets using coding classifiers such as the ICD-9. The ICD-9 is more pathologically based compared with the clinically oriented ICD-10.Reference Roozenbeek, Maas and Menon 109 Thus TBI without overt pathologic features would not be captured. As such, identification of mild TBI using ICD-9 codifiers results in high false-negative results,Reference Bazarian, Veazie, Mookerjee and Lerner 110 although the system still has reasonable sensitivity and specificity for the identification of severe TBI.Reference Carroll, Cochran, Guse and Wang 111 In addition, ICD codes are developed primarily for administrative purposes, and thus their use in epidemiological studies is convenient, but not ideal. Many of the studies only identified cases of TBI presenting to hospital. The majority of patients mild head injuries do not present to hospital, and those with severe injuries that die before presentation are not registered. This results in gross underestimation of the number of head injuries that do not present to medical professionals.
Perhaps the largest source of heterogeneity lies in the lack of a universal system for diagnosis and classification of TBI. Many studies use alterations in GCS scale as a means to diagnose TBI; however, not all TBIs present with changes in GCS. This specifically complicates the identification of mild TBI and studies in which telephone surveys and questionnaires were used. Language surrounding the definition of mild TBI is ambiguous, and physicians and laypeople use the terms head injury, brain injury, and concussion interchangeably without awareness of a discrete definition. In fact, there are approximately 41 systems described for the diagnosis and classification of mild TBI.Reference Peloso, Carroll, Cassidy, Borg, von Holst and Holm 112 Moreover, many health care authority bodies have independently published definitions for mild TBI. For example, The American Congress of Rehabilitation Medicine defines mild TBI as an injury resulting from “traumatically induced physiological disruption of brain function,” 113 whereas the World Health Organization describes mild TBI as “an acute brain injury resulting from mechanical energy to the head from external forces.”Reference Carroll, Cassidy, Holm, Kraus and Coronado 114 The American Congress of Rehabilitation Medicine definition restricts mechanism of injury only to indirect trauma to the head, and the World Health Organization definition excludes any injuries involving drugs, alcohol, medications, or addition of other systemic injuries. Thus, it is apparent that both definitions restrict cases of TBI to a limited number of mechanisms and that the spectrum of TBI cases is captured in neither definition. The Working Group on Demographics and Clinical Assessment of the International Interagency Initiative toward Common Data Elements for Research in TBI and Psychological Health states a broader definition of TBI, stating that it is “an alteration in brain function or other evidence of brain pathology caused by an external force.”Reference Menon, Schwab, Wright and Maas 115 Despite this, vague terms such as “alteration in brain function” complicate the identification of cases, because confounders such as pain and medications can certainly cause alterations in brain function with or without brain injury. The use of diagnostic imaging studies for the identification of TBI is also problematic because commonly used techniques such as computed tomography scan overlook mild injuries. Thus, until an internationally accepted definition of TBI is developed, coupled with a universal diagnostic algorithm, epidemiological studies of TBI will be fraught with discrepancies.
Conclusion
In summary, we conclude that mild TBI is more common than moderate or severe TBI, and that the incidence of TBI is higher in males and lower at the extremes of age (i.e. pediatric or elderly). Furthermore, the incidence of brain injury seems to vary widely across countries but cross-country comparisons are difficult because of the significant heterogeneity between studies and the small number of studies that could be meta-analyzed. Despite being an important medical, economic, and social problem, the epidemiology of brain injury is not well-characterized in the current literature, and capturing the incidence of brain injury remains a challenge, particularly with mild TBI. Future areas of research should focus on standardizing epidemiological studies, reporting measures, and diagnostic criteria in brain injury. For example, future epidemiologic studies in TBI could focus on methods such as door-to-door studies or surveys, which have the advantage of capturing cases of brain injury that do not present to hospital. Thus, we presume that the true incidence of brain injury is likely greater than what is currently reported. Continued research into the epidemiology of this disease is necessary in order to inform evidence-based health care, prevention, treatment, and rehabilitation initiatives.
Acknowledgments and Funding
This study is part of the National Population Health Study of Neurological Conditions. We wish to acknowledge the membership of Neurological Health Charities Canada and the Public Health Agency of Canada for their contribution to the success of this initiative.
Funding for the study was provided by the Public Health Agency of Canada. The opinions expressed in this publication are those of the authors/researchers, and do not necessarily reflect the official views of the Public Health Agency of Canada.
Disclosures
NJ has served as a principal investigator and received a shared research grant from Hotchkiss Brain Institute and Pfizer; served as a principal investigator for Canada Research Chair Program and Alberta Innovates Health Solutions; holds a Canada Research Chair Tier 2 in Neurological Health Services Research and held an Alberta Innovates Health Solutions Population Investigator Award during part of this study; salary support from a government agency and a provincial health research funding agency has been paid to NJ’s university. ADF has received an AIHS research studentship. KMF received an AIHS research studentship. The remaining authors have nothing to disclose.
Supplementary Materials
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/cjn.2016.290