Dementia is the single greatest cause of neurological disability in our senior population, with a global incidence of 47.5 million cases. 1 , 2 The annual cost of caring for dementia in Canada is $15 billion—a figure expected to grow to nearly $153 billion by 2038—including a tenfold increase in demand for long-term care.Reference Herrmann, Harimoto, Balshaw and Lanctot 3 The cumulative global cost of Alzheimer’s disease (AD) and dementia is currently estimated to be $605 billion, which is equivalent to 1% of the entire world’s gross domestic product. This figure will continue to grow unless disease-modifying interventions are found to alter these alarming trends. 1 , Reference Herrmann, Harimoto, Balshaw and Lanctot 3 Key to any such initiative will be the ability to identify early and accurately those individuals who are at risk for developing a dementia, either as an independent disease process or as a comorbidity of a related neurodegenerative or neurovascular disorder.
Here, we describe the Ontario Neurodegenerative Disease Research Initiative (ONDRI), a prospective cohort study that will use a multimodal approach to predicting the occurrence or progression of cognitive or neuropsychological impairment in a defined patient population. Disease progression will be monitored in 600 patients from across Ontario, Canada, for up to 3 years using rigorous evaluations across multiple assessment platforms, including neuroimaging, detailed neuropsychological evaluations (including comprehensive speech and language assessments), genomics, evaluations of cognitive control of eye movements and retinal layer thickness and morphology, gait performance, and neuropathology.
Patients will be enrolled in the study with either (1) AD or amnestic single or multidomain mild cognitive impairment (MCI), (2) amyotrophic lateral sclerosis (ALS), 3) frontotemporal dementia (FTD), (4) Parkinson’s disease (PD), or (5) vascular cognitive impairment (VCI). These disorders were selected based on their prevalence within the aging population and the frequent occurrence of neuropsychological dysfunction in each at various stages over their course. Amongst these, AD pathology is the most common, underlying approximately 63% of all dementia cases.Reference Herrmann, Harimoto, Balshaw and Lanctot 3 , Reference Sperling, Aisen and Beckett 4 Although objective memory loss characterizes amnestic MCI, unlike AD, the cognitive impairment does not significantly disrupt daily functioning.Reference Gomersall, Astell, Nygard, Sixsmith, Mihailidis and Hwang 5 Although traditionally considered to be a disorder of upper and lower motor neurons, more than 50% of ALS patients will exhibit neuropsychological deficits that range from subtle syndromes of cognitive or behavioural dysfunction, including impairments in social cognition, to FTD.Reference Strong, Grace and Freedman 6 When present, frontotemporal dysfunction in ALS is associated with a reduction in survival by approximately a year.Reference Woolley and Strong 7 PD affects approximately 1 in 1000 in the general population and 1 in 100 individuals older than age 65 years.Reference Dorsey, Constantinescu and Thompson 8 The prevalence of PD is expected to double by the year 2030.Reference Dorsey, Constantinescu and Thompson 8 Similar to ALS, a significant proportion of PD patients present with or will develop neuropsychological dysfunctionReference Aarsland, Zaccai and Brayne 9 and, over time, dementia (PD dementia) develops in more than 80%.Reference Hely, Reid, Adena, Halliday and Morris 10 FTD, including subtypes of primary progressive aphasia, progressive nonfluent aphasia, semantic dementia, and behavioural variant FTD, accounts for 20% of early-onset dementia cases with symptom onset commencing at 45 to 65 years of age.Reference Kertesz 11 Finally, VCI is the second most common form of dementia, with 30% of stroke patients developing dementia.Reference Saposnik, Cote and Rochon 12 – Reference Pendlebury, Mariz, Bull, Mehta and Rothwell 14 The risk of stroke and dementia rises exponentially with each decade after age 65, with one-third of our population expected to have a stroke, dementia, or both in their lifetime.Reference Hachinski, Iadecola and Petersen 15
We hypothesize that this multimodal approach will rapidly detect meaningful neuropsychological change over a short interval, allowing for the early and accurate prediction of the presence of, or progression of, dementia. We further hypothesize that we will identify different forms or profiles of dementia that will map onto specific neural circuits, some shared and some differing across disorders depending on the hubs primarily affected. Further, the study will examine the contribution of small-vessel pathology to each of these disorders given the increasing recognition of the prevalence and potential synergistic effects of this important comorbidity. In this paper, we describe the core components of ONDRI.
Methods
Participants
Six hundred participants who have one of the following diseases: AD/MCI (150: 75 AD; 75 MCI); ALS (90); FTD (60); PD (150); or VCI (150) will be enrolled into this longitudinal study from multiple centres throughout Ontario (Figure 1). Inclusion and exclusion criteria for the study as a whole, for each disease entity, and for each platform are delineated in the Supplementary material. Ethics approval was obtained from all participating institutions. Each participant will have a study partner and will be evaluated using multiple assessments for a total of 7 to 8 hours annually (unless otherwise specified) (Table 1).
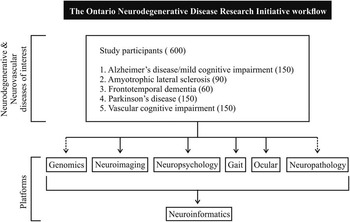
Figure 1 The Ontario Neurodegenerative Disease Research Initiative workflow. Dashed arrows represent single time visits.
Table 1 ONDRI-specific platforms, modalities, assessment, and requirements of participants
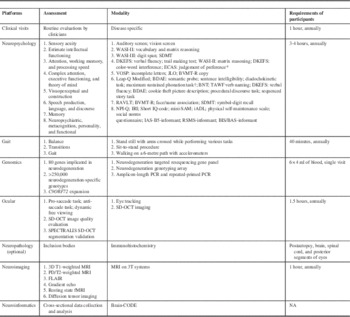
BDAE, Boston diagnostic aphasia examination; BIS/BAS, behavioural inhibition system/behavioural activation system; BNT, Boston naming test; BVMT-R, brief visuospatial memory test-revised; DKEFS, Delis-Kaplan executive function system; ECAS, Edinburgh Cognitive and Behavioural ASL Screen; FLAIR, fluid attenuation inversion recovery; fMRI, functional MRI; iADL, instrumental activities of daily living; IAS, interpersonal adjective scales; IRI, interpersonal reactivity index; JLO, judgement of line orientation; MRI, magnetic resonance imaging; NA, not applicable; NPI-Q, neuropsychiatric inventory questionnaire; PD/T2, proton density; RAVLT, Rey auditory verbal learning task; RSMS, revised self-monitoring scale; SAM, survey of autobiographical memory; SDMT, symbol-digit modalities test; SD-OCT, spectral domain optical coherence tomography; T (imaging), Tesla; TAWF, test of adolescent/adult word finding; VOSP, visual object and space perception battery; WASI, Wechsler abbreviated scale of intelligence, 2nd edition.
* Only given to the ALS and FTD cohort.
† Not given to the AD/MCI cohort.
For each platform, the respective assessment tools are listed in Table 1. To be eligible for the study, each patient must be capable of completing each component, with the exception of the neuropathology. In addition to the platforms, each patient will complete several clinical measures annually, including a neurological examination, a cognitive screen (Montreal Cognitive Assessment), vital signs, neuropsychiatric, quality of life, sleep, and disability impact questionnaires, and, where applicable, functional rating scales (for example, Movement Disorder Society Unified Parkinson’s Disease Rating Scale, National Institutes of Health Stroke Scale (NIHSS) for VCI patients, ALS functional rating scale-revised for the ALS patients). At baseline, comprehensive demographic information will also be collected, and a detailed medical and family history obtained. Patients with FTD will also undergo electrophysiological studies at baseline and at 1 year to document the presence or absence of lower motor neuron dysfunction. For the ALS cohort, participants will complete a neuromuscular examination and forced vital capacity evaluation at baseline and at follow-up visits. In addition to their annual visits, participants will complete a 6-month telephone visit that will include the clinical questionnaires and the telephone version of the Montreal Cognitive Assessment.
Neuropsychology
Eight neurocognitive domains will be evaluated (Table 1 and Supplementary material). These domains are broad-based and include attention, processing speed, memory, speech production, language, intelligence, and visuospatial function, with a particular focus on cognitive domains that reflect frontal network functioning, including complex attention, executive functions, and social cognition. Furthermore, in accordance with the goal of discerning the effect of vascular disease on each neurodegenerative disease under study, the composition of tests reflects the recommendations of the VCI harmonization standards.Reference Pendlebury, Mariz, Bull, Mehta and Rothwell 14 Participants and their study partners will complete a series of questionnaires that provide measures of neuropsychiatric functioning, meta-cognitive skills, personality, and activities of daily living. The neuropsychological evaluation is being conducted in all participants annually, with the exception of ALS participants who will be evaluated biannually given the nature of the disease and its rapid progression.
Gait
A standardised protocol for assessing balance and gait under single and cognitive demanding dual-task conditions will be used. Static balance (stationary standing) will be assessed using wireless force board technology and accelerometry under eyes open and closed visual conditions during 30-second trials. Estimates of centre of pressure and centre of mass sway will be computed. Gait, which demands dynamic stability, will be assessed using wearable accelerometry (Gulf Coast) bilaterally at ankle and hip or an electronic walkway (GAITRite System or PKMas System). Gait will be assessed under two task conditions: (1) preferred walking alone (single task) and (2) dual-task walking performing a concurrent secondary cognitive task. The secondary tasks include: (a) counting backwards from 100 by ones, (b) subtracting serial sevens from 100, and (c) naming animals.Reference Montero-Odasso, Casas and Hansen 16 All cognitive tasks will be performed out loud and no instruction to prioritize the gait or cognitive task will be provided, allowing both gait and cognitive tasks to vary naturally. Gait characteristics (e.g. velocity, cadence, step length, step and stride time variability) and dual-task gait cost (%) will be calculated. Dual-task cost will be calculated as ([single-task gait value – dual-task gait value]/simple-task gait value)×100.Reference Montero-Odasso, Muir and Speechley 17 Step/stride time variability will be calculated as the coefficient of variation for stride time: Coefficient of variation=(standard deviation of stride time/mean stride time)×100.
Genomics
Each ONDRI participant will be screened for mutations within a panel of neurodegenerative disease genes that was selected, curated, and reviewed by neurodegeneration researchers (Table 1). Specifically, next-generation sequencing technology is being deployed to identify any genetic variation using ONDRISeq.Reference Farhan, Dilliott and Ghani 35 In conjunction, we are genotyping all participants for >250,000 single-nucleotide polymorphisms (SNPs) associated with neurodegeneration as represented on the NeuroX chip.Reference Ghani, Lang and Zinman 18 Apolipoprotein E ε4 allele carrier status, which is a risk factor for not only AD but possibly for poststroke dementia and PD dementia, is being evaluated.Reference Ballard, Morris and Rao 19 , Reference Tsuang, Leverenz and Lopez 20 All ONDRI participants will be genotyped for the C9ORF72 expansion using amplicon length polymerase chain reaction analysis and repeat-primed polymerase chain reaction as previously described.Reference Xi, Zinman and Grinberg 21
Ocular Assessments
For the ocular platform, individuals with glaucoma will be excluded.
Ocular I: Eye Tracking
All eye tracking will be conducted with a portable Eyelink 1000 Plus eye tracker (SR Research, Ottawa, Canada). Participants will perform three specific tasks including a pro-saccade and an anti-saccade task. In the former, simple sensory motor function will be evaluated by the patient looking from a central fixation point to the target, thus eliciting a highly automatic response that is used to determine the shortest latency of visually triggered saccades and saccade metrics to assess the integrity of the visual and motor pathways. In the latter, the participant will be instructed to look away from the stimulus. This task dissociates sensory and motor processes and measures flexible behavioural control. Participants must suppress the automatic pro-saccade, invert the target vector, and then generate a voluntary anti-saccade.
Dynamic free viewing will be used to evaluate the relative components of top-down and bottom-up attention to processing scan paths during unstructured free viewing of video clips. The participant will view video clips that switch randomly every 2 to 4 seconds from one scene or event to another (this is similar to watching television where the channel changes are out of their control). Every time the clip changes, the top-down system (willed processes) loses the ability to guide the next two to three saccades and the bottom-up system (reflective processes) takes over.
Ocular II: Spectral Domain Ocular Coherence Tomography
The Heidelberg Spectralis spectral domain ocular coherence tomography (SDOCT) Blue Peak instrument (Heidelberg Engineering GmbH, Heidelberg, Germany) will be used to quantify retinal nerve fibre layer thickness. This instrument allows for infrared, fundus autofluorescence, and SDOCT imaging and will include Nsite Axonal Analytics software. The Spectralis uses a 870-nm center wavelength super luminescent diode and it can acquire up to 40,000 A-scans/second with a depth (z-plane) resolution of 7 μm and a transversal (x, y-plane) resolution of 14 μm in the retina. The number of A-scans, or pixels, within the image determines the transversal resolution and this can be adjusted by the operator. High-resolution mode provides 5 μm transversal resolution, whereas high-speed mode provides 11 μm resolution because the distance between A-scans is doubled. An online eye-tracking device decreases motion artifacts, whereas the high scanning speed reduces the impact of involuntary eye movements. Two Spectralis SDOCT scan protocols (retinal nerve fibre layer-N scan protocol; posterior pole scan protocol) will be used and three images will be acquired for each of these protocols.
Neuroimaging
All ONDRI magnetic resonance imaging (MRI) scans will be performed at a magnetic field strength of 3 Tesla. Ten MRI centres across the province have been approved for scanning subjects in the ONDRI study (five Siemens scanners, four General Electric scanners, one Philips scanner). Each MRI site performs monthly quality control assessments incorporating scanning the Functional Bioinformatics Research Network (FBIRN) phantomReference Friedman, Stern, Brown, Mathalon, Turner and Glover 22 and a gradient distortion correction phantom. Six different MRI protocols (Table 1) are run on each subject in the following order: three-dimensional T1-weighted anatomical scan (1 mm isotropic resolution) used for volumetric assessment of brain structures, proton density (PD)/T2-weighted scan (resolution time [TR]=3000, echo time 1 [TE1] ~10 ms, TE2 ~90-100 ms, 3 mm thick interleaved) used for the assessment of tissue ischemia and skull stripping, fluid-attenuated inversion recovery (TR=9000 ms, TI ~2250-2500 ms) for the assessment of white matter hyperintensities, gradient echo (TR=650 ms, TE=20 ms) for the assessment of tissue microbleeds, resting state functional MRI (TR=2400 ms, TE=30 ms, flip angle=70°, 3.5 mm isotropic resolution, 250 volumes, 10-minute acquisition time) for the evaluation of brain network activity, and finally diffusion tensor imaging (2 mm isotropic resolution, 30 to 32 directions, b-value=1000) for the evaluation of white matter structural integrity. The total time required for all imaging is approximately 45 to 50 minutes. All images are uploaded to the Brain-CODE database and undergo rigorous quality control to assess protocol adherence and evaluate signal-to-noise ratio, contrast-to-noise ratio, and the presence of image artefacts.
An initial volumetric analysis will be made by the imaging platform and the results provided to investigators. These analyses will include volumetric analysis of hippocampal volume,Reference Nestor, Gibson, Gao, Kiss and Black 23 ventricle volume,Reference Nestor, Rupsingh and Borrie 24 as well as 26 different volumes of interest using Signal amplification by reversible exchange (SABRE)Reference Dade, Gao and Kovacevic 25 and Lesion Explorer.Reference Ramirez, Gibson and Quddus 26 Additional measurements are planned to include number of microbleeds, regional measurements of diffusional fractional anisotropy and mean diffusivity, and resting-state network connectivity.Reference Wahlund, Julin, Lindqvist and Scheltens 27 - Reference Cordonnier, Potter and Jackson 31
The structural images are reviewed by a board-certified neuroradiologist to detect any incidental findings or exclusion pathology.
Neuropathology
Neuropathology provides the diagnostic gold standard against which the clinical and neuroimaging diagnoses are tested. Such confirmation has not yet been replaced by other diagnostic approaches because recent data indicate that more than 10% of patients entering clinical trials for treatment of AD suffer from other conditions.Reference Holmes, Boche and Wilkinson 32 Beyond the categorical diagnosis, the neuropathological assessment will also document the extent to which multiple neurodegenerative pathologies are present over and above the primary diagnosis. The extent of concomitant vascular disease will also be evaluated. Autopsies will be performed at the local sites, and the neuropathological diagnosis rendered would be entered in the history. Fixed or paraffin-embedded tissue blocks, as well as a limited sample of frozen brain tissue, will be sent to a central laboratory where they will be subjected to uniform staining. After scanning the slides, evaluation will be performed by two different neuropathologists and the diagnosis and quantitative assessment of lesion load will be entered into the database. In this way, the clinical and research use of the autopsy is entirely separate. The fixed and frozen tissue samples will also be made available for further analyses (e.g. genomic, epigenetic, proteomic, metabolomics research to study the molecular pathology underlying neurodegeneration). In addition, the digital slides constitute an inexhaustible resource that can be distributed to as many researchers as necessary.
Neuroinformatics
All data collected across all assessments will be deidentified and uploaded into a central database, Brain-CODE, including MRI and SDOCT images, and will be analyzed across modalities and cohorts using mixed models to identify common and unique predictors of cognitive decline across the five neurodegenerative diseases. Where appropriate, we will use propensity-weighted multivariate regression analysis (which accounts for different sources of selection bias in observational studies) and also partial least squares regression (given limited sample size and multicollinear variables in some analyses).
Discussion
ONDRI is a unique, prospective multimodality study to improve understanding of the pathogenesis of neuropsychological deficits across a broad range of neurodegenerative disorders (Figure 2). Beyond this, ONDRI has already facilitated the development of standardized assessment protocols, which will allow for direct comparisons to be made across a range of neurodegenerative and neurovascular disorders. These tools will allow for a systems-based approach to identify not only unique clinical, imaging, ocular, genetic, and other biological markers associated with each of these disorders, but also to define where there are significant biological overlaps. Although the numbers of patients being studied is small (600) relative to larger prospective studies, patients enrolled in ODNRI will be a unique resource because they will be deeply endophenotyped and their deidentified data publicly available by request through the ONDRI publications and data analysis committee.
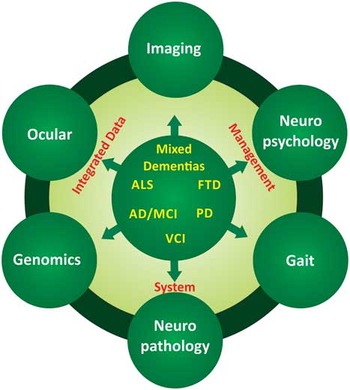
Figure 2 Schematic illustration of the interrelationship amongst the evaluative platforms and disease processes being studied in the Ontario Neurodegenerative Disease Research Initiative.
Current therapeutic approaches in neurodegenerative diseases, where available, tend to be directed towards single biological mechanisms that may be inadequate given the complexity of these multifaceted diseases in addition to the spectrum of mechanisms by which neurodegeneration is expressed.Reference Iadecola and Anrather 33 Through this integrated discovery approach, we have the unique opportunity to identify multiple markers of brain health that will contribute to the development of: (1) biomarkers for neurodegenerative diseases that may ultimately be used in the identification of presymptomatic individuals; (2) improved identification of overlap syndromes amongst neurodegenerative diseases for both clinical and research purposes; and (3) personalized treatments, which may require a “cocktail” similar to the approach that has been applied in cancer, and are likely to be more efficacious in halting disease progression than current drug regimens.Reference Lang 34 These may vary depending on the stage of the disorder being treated, the genetic predispositions discovered to be contributing to an individual’s disease, and the possible role of mixed pathologies. Hence, this well-structured and integrated longitudinal exploratory study has the potential to contribute to significant effects on health care in the rapidly growing area of neurodegenerative and neurovascular diseases.
Finally, the nature of ONDRI is such that the data obtained will be made available to the wider scientific community as well as ultimately federated with data arising from a range of other studies currently examining neurodegenerative disorders, including comparative cohort studies just getting underway in Canada such as the Brain Eye Amyloid Memory study (www.tdra.ca) and the Canadian Consortium on Neurodegeneration in Aging (www.ccna-ccnv.ca).
Acknowledgements and Funding
The authors thank and acknowledge the consent and cooperation of all ONDRI participants. Many thanks to the ONDRI investigators (lead investigator: Michael Strong) and the ONDRI governing committees: executive committee; steering committee; publication committee; recruiting clinicians; assessment platforms leaders; and the ONDRI project management team. For a full list of the ONDRI investigators, please visit: www.ONDRI.ca/people.
The ONDRI project is funded by the Ontario Brain Institute through the Government of Ontario with matching funds provided by participating hospital and research institute foundations, including the Baycrest Foundation, Bruyère Research Institute, Centre for Addiction and Mental Health Foundation, London Health Sciences Foundation, McMaster University Faculty of Health Sciences, Ottawa Brain and Mind Research Institute, Queen’s University Faculty of Health Sciences, Providence Care (Kingston), Sunnybrook Health Sciences Foundation, the Thunder Bay Regional Health Sciences Centre, the University of Ottawa Faculty of Medicine, and the Windsor/Essex County ALS Association. The Temerty Family Foundation provided the major infrastructure matching funds. SMKF is supported by the Canadian Institutes of Health Research Frederick Banting and Charles Best Canada Graduate Scholarship. RHS receives salary support from the Heart and Stroke Foundation of Canada New Investigator and Henry J.M. Barnett Awards, the L.C. Campbell Research Chair. and the Canadian Partnership for Stroke Recovery.
Statement of Authorship
RB, SEB, DC, EF, MF, BG, DAG, RAH, CH, PWK, AEL, MM, PMM, MM-O, DGM, DPM, S. Strother, RHS, S. Symons, MCT, LZ, and MJS conceived and designed the study. SMKF, RB, SEB, DC, EF, MF, BG, DAG, RAH, CH, PWK, AEL, MM, PMM, MM-O, DGM, DPM, S. Strother, RHS, S. Symons, MCT, LZ, and MJS prepared the manuscript. MJS was the study lead investigator.
Disclosures
The authors do not have anything to disclose.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2016.415





