Multiple system atrophy (MSA) is a sporadic neurodegenerative disease involving autonomic dysfunction with parkinsonism or cerebellar ataxia.Reference Nadia, Philipp and Susanne 1 With the identification of α-synuclein-positive glial cytoplasmic inclusions as a pathological hallmark, MSA was thought as a unique entity within the spectrum of oligodendrogliopathyReference Wenning, Stefanova and Jellinger 2 ; however, the etiology of MSA is not clear. Recently, mitochondrial dysfunction and oxidative stress have been considered as risk factors of triggering or exacerbating MSA pathology.Reference Stefanova, Reindl and Neumann 3 , Reference Ubhi, Lee and Adame 4 Some oxidant biomarkers, such as uric acid and lipids, have been considered as potential predictors for the prevalence of MSA.Reference Cao, Guo and Chen 5 , Reference Cao, Guo and Chen 6
In addition to uric acid and lipids, serum homocysteine (Hcys) and bilirubin are also related with oxidative stress. Hcys plays an important role in some neurodegenerative diseases associated with oxidative stress, such as Alzheimer’s diseaseReference Seshadri, Beiser and Selhub 7 , Reference Schalinske and Smazal 8 and Parkinson’s disease (PD).Reference Schalinske and Smazal 8 , Reference O’Suilleabhain, Sung and Hernandez 9 A study also found elevated plasma Hcys levels may predict the outcome of disease severity in PD patients.Reference Zhang, Yan and Xu 10 Inversely, bilirubin contributes to defend against the increased oxidative stress. Some studies have demonstrated low bilirubin levels and oxidative stress occurred in some neuroinflammatory diseases and neurodegenerative diseases.Reference Vítek 11 - Reference Iłzecka and Stelmasiak 13 Additionally, some environmental factors, especially various occupations, toxin, smoking, drinking, different living area, dietary habits, and use of drugs, were also related to oxidative stress and the progression of MSA.Reference Nee, Gomez and Dambrosia 14 - Reference Seo, Yong and Song 18
In past decades, Hcys and bilirubin have been explored in numerous neurological diseases; however, these two biomarkers and MSA remain unclear. The aim of this study was to examine serum Hcys, bilirubin, uric acid, lipids, and potential environmental risk factors and to ascertain whether these data correlate with MSA in a Chinese population.
Methods
Clinical Data Collection and Definitions
We retrospectively reviewed the medical records of 55 MSA patients (37 males and 18 females) according to the diagnostic criteria of clinical types, probable MSA and possible MSAReference Gilman, Wenning and Low 19 from January 2007 to November 2013 admitted to the Third Affiliated Hospital of Sun Yat-sen University in Guangzhou, China. Seventy-six age- and sex-matched healthy subjects (42 males and 34 females) as the healthy controls were consecutively recruited from the Medical Examination Center, the Third Affiliated Hospital of Sun Yat-sen University. The exclusion criteria were listed as: (1) received vitamin supplementation including folic acid, vitamin B12, and vitamin B6 in the past 5 years; (2) having liver or gall diseases; (3) history of neurodegenerative diseases other than MSA; (4) having any other clinically significant medical illnesses; or (5) pregnancy. Data collection was approved by the institution’s ethics committee of the Third Affiliated Hospital of Sun Yat-sen University. All people in this study have given their informed consent before inclusion.
Data of age, sex, education level, anti-parkinsonian agent usage, and illness duration as well as the exposure factors, including smoking habits, drinking habits, farming history, and rural living history, were collected. The disease severity of MSA patients was assessed by The Unified MSA Rating Scale I (UMSARS-I). Every one of the 12 items including speech, swallowing, handwriting, cutting food and handling utensils, dressing, hygiene, walking, falling, orthostatic symptoms, urinary function, sexual function and bowel function possessed 4 points in UMSARS-I scoring, making the max scores as 48.Reference Wenning, Tison and Seppi 20 The Hoehn & Yahr (H&Y) stage and International Cooperative Ataxia Rating Scale (ICARS) were used to evaluate the parkinsonism and ataxia of the patients respectively. The cognitive condition of each subject was determined by Mini-Mental State Examination (MMSE).
Serum Hcys, Bilirubin, Uric Acid, and Lipids Determination
The concentrations of serum Hcys (normal range, 3.7-13.9 μmol/L), total bilirubin (Tbil) (normal range, 4.0-23.9 μmol/L), indirect bilirubin (Ibil) (normal range, 2.56-20.9 μmol/L), uric acid (normal range, 90-420 μmol/L), and lipid including total cholesterol (TC) (normal range, 3.10-5.70 mmol/L), triglyceride (normal range, 0.34-1.92 mmol/L), high-density lipoprotein cholesterol (HDL-C) (normal range, 0.78-2.00 mmol/L), and low-density lipoprotein cholesterol (LDL-C) (normal range, 2.07-3.10 mmol/L) were determined in our hospital. Blood samples were obtained at rest and after a 12-hour fasting period.
Statistical Analysis
All statistical analyses were performed by the Statistical Program for Social Sciences (SPSS) statistical software (version 13.0, Chicago, IL, USA). All the data of continuous variables, including age, illness duration, UMSARS-I, H&Y, ICARS, MMSE, Hcys, Tbil, Ibil, uric acid, TC, triglyceride, HDL-C, and LDL-C were presented as mean ± standard deviation and the categorical data such as sex, education level, dopaminergic medication usage, smoking habits, drinking habits, farming history, and rural living history were shown as percentages. Statistical significance was set at p<0.05. Student’s t-test or one-way analysis of variance was performed to determine the differences in continuous data between MSA patients and the controls, and chi-square test was performed for categorical variables. A Spearman’s correlation coefficient was used to determine the associations between Hcys, bilirubin, uric acid, lipids, and the clinical variables, including illness duration, UMSARS-I, H&Y, ICARS, and MMSE. Logistic regression was used to analyze exposure data (smoking habits, drinking frequency, farming history, and rural living history) and to determine odds ratios (OR) and 95% confidence intervals (OR [95% CI]).
Results
Patient Characteristics
Fifty-five MSA patients including 39 probable MSA and 16 possible MSA (37 males [67.3%] and 18 females (32.7%]) and 76 healthy control subjects (42 males [55.3%] and 34 females [44.7%]) were enrolled in this study (Table 1). The MSA subjects were categorized as MSA-P (23 [41.8%]) and MSA-control (32 [58.2%]), and 20 MSA patients (36.4%) received anti-parkinsonian agents (Table 1). As shown in Tables 1 and 2, there were no significant differences in mean ages, sex distribution, and education levels between MSA patients and controls. Exposure factors before the onset of the disease in the MSA group and the index age in the control group are listed in Table 4; the exposures at the admission time are shown in Table 5.
Table 1 Sex distribution, education level, clinical type, and anti-parkinsonian agent usage of MSA patients and controls

All 20 MSA patients treated with anti-parkinsonian agents adopted the treatment of levodopa (0.375-1.000 g/day); four patients received a combination with dopamine agonists (two with pramipexole [0.375 and 0.75 mg/day], two with piribedil [50 and 100 mg/day]), two patients received a combination with amantadine (100 mg/day), and one patient received a combination of anticholinergic agents (4 mg/day).
Table 2 Age, Hcys, bilirubin, uric acid, lipid, illness duration, UMSARS-I, H&Y, ICARS, and MMSE in MSA and controls

The mean illness duration of MSA patients was 2.62±2.20 years; the mean UMSARS-I scores were 10.92±4.40. The H&Y stage of MSA patients was significantly higher than that of the controls (1.53±1.42 vs 0.36±0.64, p<0.001). The MMSE mean scores of MSA patients were significantly lower than those of controls (24.70±5.45 vs 28.14±2.00, p<0.001) (Table 2).
Comparison of Serum Hcys, Bilirubin, Uric Acid, and Lipid Levels Between MSA Patients and Controls
Serum Hcys levels in MSA patients were higher than those in the controls (13.52±4.56 vs 10.28±3.31 μmol/L, p<0.001) (Table 2). Serum levels of bilirubin including Tbil and Ibil were lower in MSA patients than those in controls (11.61±4.21 vs 13.88±5.30 μmol/L, p=0.007; 8.28±3.05 vs 9.86±3.98 μmol/L, p=0.011, respectively). There were no significant differences in the uric acid levels between MSA patients and controls. In the lipids data, only TC levels in MSA were significantly lower than those in controls (4.45±1.22 vs 4.99±1.69 mmol/L, p=0.046).
Associations Between Serum Hcys, Bilirubin, Uric Acid, Lipids, and Disease Severity of MSA
Significant correlations between Hcys and illness duration/UMSARS-I (r s =0.422, p=0.001; r s =0.555, p<0.001, respectively) occurred in MSA patients. And HDL-C levels had a negative correlation with UMSARS-I (r s =-0.325, p=0.015) and a positive correlation with H&Y (r s =0.398, p=0.003). LDL-C levels also had positive correlations with ICARS (r s =0.281, p=0.037) and MMSE (r s =0.303, p=0.024). There were no associations between serum bilirubin/uric acid and disease severity of MSA (Table 3 and Fig. 1).

Figure 1 Correlation between the serum oxidative biomarkers and illness duration, UMSARS-I, H&Y, ICARS, and MMSE in MSA patients. (A) Hcys levels have a positive association with illness duration. (B) Hcys levels are positively associated with UMSARS-I. (C) Serum HDL-C has a negative correlation with UMSARS-I. (D) Serum HDL-C negatively correlates with H&Y stage. (E) Serum LDL-C positively correlates with ICARS scores. (F) Serum LDL-C positively correlates with MMSE scores.
Table 3 Correlation between Hcys, bilirubin, uric acid, lipids, and clinical variables, including illness duration, UMSARS-I, H&Y, ICARS, and MMSE in MSA patients

Exposed Factors in MSA Patients
Current smoking was less frequent in MSA patients than in controls (adjusted OR, 0.13; 95% CI, 0.03-0.48; p=0.002). And farming was more frequent in MSA patients than in controls (1-20 years: adjusted OR, 6.36; 95% CI, 2.36-17.12; p<0.001; >20 years: adjusted OR, 10.26; 95% CI, 2.68-39.30; p=0.001). A significant difference between MSA patients and controls occurred in the rural living analysis (crude OR, 2.91; 95% CI, 1.35-6.27; p=0.006), but that disappeared after adjustment for other factors (Table 4). There were no significant associations between UMSARS-I and these exposed factors including smoking, drinking, duration of farming, and rural living history at the admission time (Table 5).
Table 4 Smoking, drinking, and farming and rural living exposures (at the onset of MSA) of MSA patients and controls

Without statistical analysis results. Adjusted OR indicates odds ratios adjusted for sex, age and education level;
a Adjusted for drinking, farming, and rural living.
b Adjusted for smoking, farming, and rural living.
c Adjusted for smoking, drinking, and rural living.
d Adjusted for drinking, smoking and farming.
Table 5 Association between different exposure factors (at admission time) and UMSARS-I in MSA patients
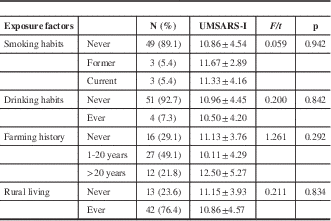
F=statistics for smoking habits and farming history; t=statistics for drinking habits and rural living.
Discussion
More and more clinical data and experimental studies have considered whether oxidative stress would play an important role in the pathogenesis of MSA.Reference Stefanova, Reindl and Neumann 3 - Reference Cao, Guo and Chen 6 Oxidative stress can induce neuronal damage, modulate intracellular signaling, and ultimately lead to neuronal death by apoptosis or necrosis.Reference Calabrese, Cornelius and Mancuso 21 Our research showed that Hcys levels in MSA were significantly higher than those in the controls, whereas serum levels of Tbil and Ibil were lower in MSA patients. Hcys is a risk factor for neurotoxicity and leads to brain damage in humans.Reference Obeid and Herrmann 22 Elevated Hcys levels are widely reported to be associated with neurodegenerative diseases through oxidative stress.Reference Calabrese, Cornelius and Mancuso 21 , Reference Schlüssel, Preibisch and Pütter 23 , Reference Petras, Tatarkova and Kovalska 24 Bilirubin, the end-product of heme metabolism, has a stronger antioxidant capacity than many other antioxidants, including α-tocopherol, superoxide dismutase, and catalase.Reference Peng, Yang and Liu 25 Moreover, higher serum Hcys levels are associated with longer illness duration and higher UMSARS-I scores, and probably suggests that higher levels of Hcys may predict worse disease severity in MSA. However, the correlation between Hcys and illness duration/UMSARS-I is weak, which may be attributed to small sample size and other unknown factors affecting Hcys and the disease severity of MSA. Although MMSE scores of MSA patients were lower than controls, and dementia has been widely reported to correlate with higher Hcys levels,Reference Seshadri, Beiser and Selhub 7 , Reference Seshadri 26 the correlation between Hcys and MMSE in MSA patients was not shown in this study. This result suggests that Hcys is not the main factor affecting cognitive impairment in MSA, although oxidative stress exists in both dementia and MSA. Uric acid is also considered an antioxidant and has been found to play a key role in the risk and progression of some neurodegenerative diseases associated with oxidative stress, such as PD,Reference Winquist, Steenland and Shankar 27 amyotrophic lateral sclerosis,Reference Zoccolella, Simone and Capozzo 28 and MSA.Reference Cao, Guo and Chen 5 However, we did not find any significant changes in the uric acid levels in patients with MSA in this study.
In this study, the results suggest that the serum TC is significantly decreased in MSA patients. HDL-C still plays a protective role in the severity of MSA because its negative correlation with UMSARS-I scores. Increased LDL-C exerts a beneficial role in ataxia and cognition according to its positive correlations with ICARS and MMSE in MSA patients. These results were similar to the study of another groupReference Cao, Guo and Chen 6 that suggested that lower cholesterol such as TC, HDL-C, and LDL-C might increase the risk of MSA. Lower TC and HDL-C were demonstrated to increase the risk of MSA.Reference Lee, Lim and Shin 29 Cholesterol in the brain membranes may modulate the conformational state of α-synuclein or even directly as a major component of α-synuclein.Reference Fortin, Troyer and Nakamura 30 Lower cholesterol levels may lead to the abnormity of α-synuclein and contribute to the pathogenesis of MSA. Another interesting finding is that higher HDL-C has a positive correlation with H&Y stage, although it has a negative correlation with UMSARS-I scores. This is consistent with the finding of Cao et al that serum HDL-C was increased in MSA-P patients.Reference Cao, Guo and Chen 6 Additionally, HDL-C was recently reported to have positive association with the duration of PD.Reference Cassani, Cereda and Barichella 31 A possible explanation is that progressive motor impairment such as exercise may affect the HDL-C metabolism.Reference Durstine, Grandjean and Davis 32 Improved movements of PD patients were associated with a reduction in HDL-C.Reference Rieu, Pereira and Derost 33
Epidemiological studies have shown that oxidative stress played key functions in the pathogenesis of MSA. For example, farming involves a heterogeneous exposure to different chemical and biological factors (pesticides, solvents, mycotoxins, dust, fuels, oils, fertilizers, farm animals),Reference Alavanja, Sandler and McMaster 34 which might interfere with the mitochondrial electron transport chain and induce oxidative stress to trigger or exacerbate MSA.Reference Nee, Gomez and Dambrosia 14 , Reference Seo, Yong and Song 18 In this study, we also found that those involved in farming show an increased risk of MSA. And we found that current smoking appears to be less frequent among MSA patients than controls, which is similar to the European study in MSAReference Vanacore, Bonifati and Fabbrini 35 and the Spanish study in PD,Reference Allam, Del Castillo and Navajas 36 but opposite to the Korean epidemiological study.Reference Seo, Yong and Song 18 In terms of protective effects, nicotine was reported as an antioxidant, which may be intracellular through the activation of the nicotinic receptors or extracellular by acting as a radical scavenger.Reference Newman, Arendash and Shytle 37 Additionally, nicotine can also act as an agonist of neuronal nicotinic receptors, which modulates functions relevant to PD via stimulation of dopaminergic transmission in the nigrostriatal pathway.Reference Kucinski, Wersinger and Stachowiak 38 We also found that exposures did not have significant associations with UMSARS-I in MSA patients, suggesting that the environmental exposures probably did not worsen or ameliorate the disease severity, but only influenced the occurrence of MSA. In addition to these factors included, other environmental exposure factors were found to be related to MSA in other studies, such as being a machine operator and assembler and using herbal medications associated with increased risk for MSA, whereas consumption of meat, seafood, tea, and coffee and the use of antihypertensive medication, aspirin, and vitamins was associated with a decreased risk.Reference Vidal, Vidailhet and Elbaz 17 , Reference Seo, Yong and Song 18 These epidemiological data prompted the belief that some environmental factors may provide clues to the etiopathogenesis of MSA.
In summary, this study found that elevated Hcys and decreased HDL-C levels may be associated with the disease severity of MSA. Environmental exposures such as farming and smoking may contribute to the occurrence of MSA, but not the progression. The results suggest that oxidative stress is involved in the pathogenesis of MSA; however, further studies are still needed for better understanding the etiology of this disease.
Acknowledgments and Funding
This work was supported by grants (to XC) from the Israel Science Foundation-the National Natural Science Foundation of China joint program (813111290) and the Natural Science Foundation of Guangdong Province (No. 2014A030313172). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LZ and YJ contributed equally to this work.
Disclosures
The authors declare there are no conflicts of interest.








