Introduction
The most effective salvage therapy in pediatric-refractory or relapsed brain tumors is yet to be established.Reference Ramaswamy, Remke and Bouffet1 The contemporary approach to upfront therapy for primary pediatric medulloblastoma (MB) is dependent on the patient’s age, size of post-operative residual tumor, and evidence of metastasis at diagnosis.Reference Polkinghorn and Tarbell2 This clinical risk stratification strategy for pediatric MB allocates children into either standard or high-risk groups, where the latter group of children will receive higher doses of upfront radiation. High-risk disease is characterized by the presence of children of age less than 3 years, the presence of metastasis at diagnosis, and post-operative residual disease of greater than 1.5 cm2. These children receive a higher dose of craniospinal irradiation (CSI) of 36 Gy with a posterior fossa boost.Reference Polkinghorn and Tarbell2–Reference Gajjar, Chintagumpala and Ashley4 This risk stratification strategy for upfront therapy has resulted in significant improvements in overall survival (OS) of children with MB conferring children with standard risk disease a 5-year OS nearing 80%.Reference Polkinghorn and Tarbell2 However, approximately 40% of patients with pediatric MB will experience recurrent disease and 30% will not survive into adulthood.Reference Jones, Jager and Kool5 Reports of phase I and phase II studies for recurrent pediatric MB exist, but the therapies provided have been observed to bear significant treatment-related morbidity and mortality.Reference Bautista, Fioravantti and de Rojas6 One such example is high-dose chemotherapy, and autologous stem cell transplant as a salvage regimen has observed prolonged survival with significant deaths (i.e., 25%) associated with treatment-related toxicities.Reference Gilman, Jacobsen and Bunin7 When the benefits of prolonged survival outweigh the risk of the treatment, the hopes for treatment response leave patients and families with little other options.
While there are reports of long-term survivors who have received differing strategies of salvage therapy to treat recurrent MB, no one strategy has proved to be superior to another.Reference Kramer, Pandit-Taskar and Humm8–Reference Crafts, Levin, Edwards, Pischer and Wilson15 With current efforts to identify driver genes for biologically targeted therapies, the heterogeneity of the genetic mutations of these tumors may in part explain the lack of definitive results with respect to benefits relating to morbidity and mortality of early molecularly targeted clinical trials.Reference Baruchel, Sharp and Bartels16, Reference Beutler, Avoledo and Reubi17 In the past decade, the international pediatric MB community identified four distinct molecular subgroups (Wnt, Shh, Group 3, and Group 4)Reference Taylor, Northcott and Korshunov18 and described associated clinical outcomes,Reference Ramaswamy, Remke and Bouffet1, Reference Cho, Tsherniak and Tamayo19 including recurrence.Reference Ramaswamy, Remke and Bouffet20 The Wnt subgroup tumors are identified by an upregulation of the Wnt pathway and have been observed to experience the most favorable clinical course with current clinical trials designed to explore de-escalation of therapy21–23 in this subgroup. The Shh group is characterized by the aberrant activation of the Shh signaling pathway. Recent biological studies suggest these tumors can be stratified into two age-associated subgroups, based on their methylation profiles, with infant Shh (iShh) experiencing a worse prognosis.Reference Schwalbe, Lindsey and Nakjang24 Subsequent methylation profiling analysis observed that the iShh group further segregates into two distinct methylation subtypes with associated clinical outcomes. iShhI was associated with a worse prognosis (i.e., 5-year progression-free survival (PFS) of 28% vs. 75% in iShhII subgroup).Reference Robinson, Rudneva and Buchhalter25 The remaining two subgroups, Group 3 and 4 MB, are not characterized by a dominant aberrant signaling pathway, but genetic profiling has verified that they are distinct from Wnt and Shh MB, as well as each other. Group 3 MB (G3 MB) bears the worse prognosis, often metastatic at presentation and is associated with the poorest outcome survival,Reference Beutler, Avoledo and Reubi17, Reference Kool, Korshunov and Remke26 especially if the tumor cells harbor the upregulation of the MYC oncogene.Reference Taylor, Northcott and Korshunov18 Group 4 MB (G4 MB) is the most common subgroup, with an intermediate prognosis,Reference Beutler, Avoledo and Reubi17 although metastasis at presentation confers poorer clinical outcome.Reference Ramaswamy, Remke and Bouffet1
In efforts to understand treatment strategies that were historically employed in our single mid-sized Canadian pediatric academic institution, we set out to describe the salvage treatments that have been provided to children with treatment-refractory or recurrent MB and their overall outcome. In addition, with a rise in molecularly targeted therapy and observations of genetic tumor factors conferring greater risk of relapse, where possible, we have described our cohort in association with available molecular MB subgrouping data.
Methods
A retrospective chart review of pediatric patients from the McMaster Pediatric Brain Tumour Study Group (PBTSG) was conducted after approval by the Hamilton Integrated Research Ethics Board (HiREB). Patients identified for this study were those who were admitted to McMaster Children’s Hospital between 2002 and 2015 and underwent surgical resection (gross total resection [GTR] or subtotal resection [STR], debulking, or biopsy) and subsequently received a histopathological diagnosis of MB. Overall, 31 patients were identified and included in this study. All patients were followed up for at least 2 years. Data including patient demographics, treatment, and outcomes were collected from a combination of electronic health records and paper charts. Molecular subgrouping was either provided by the Clinical Laboratory Improvement Amendments (CLIA)-certified NanoString facility at Toronto Sick Kids Hospital using fresh tissue from 2016 onward or performed in the laboratory of Dr. Sheila Singh from the original tumor sample, stored in RNAlater® (Sigma, #R0901) or paraffin-embedded samples requested from the Department of Neuropathology. Ten paraffin sections per sample were deparaffinised with xylene prior to RNA extraction using the RNeasy FFPE RNA extraction kit (Qiagen, #73504) as directed by the manufacturer. RNA concentrations were measured using the NanoDrop 1000 instrument (NanoDrop), and the RNA integrity was assessed at the McMaster Illumina facility. Using the Northcott MB CodeSet,Reference Northcott, Shih and Remke27 raw NanoString counts for each gene were normalized. The normalized data were subsequently log2-transformed and used as an input for class prediction as previously described.Reference Kool, Korshunov and Remke26
Descriptive statistics were performed, with categorical variables reported as counts and percentages and continuous variables as mean with standard deviation or median with range. Kaplan–Meier analysis was performed to estimate cumulative recurrence-free and survival rates with 95% confidence intervals, and log-rank test was used for between-group comparisons. A p-value of 0.05 was considered for statistical significance. SPSS version 25.0 (www.ibm.com)28 was used for data analysis.
Results
Over the 13-year period, a total of 31 children with a diagnosis of MB were consecutively treated and included in this study. Table 1 summarizes the main patient demographics. The majority of patients were male and non-infants with a median age of 8 years (16.6 years) and median follow-up of 64.8 months (168 months). The dominant histopathological subtype was large cell anaplastic, although a significant proportion did not have the subtype specified. Eight patients underwent pre-operative cerebrospinal fluid (CSF) analysis to identify CSF-seeding of the tumor with all of the results reported as negative or indiscernible. Of the 24 patients who underwent a lumbar puncture 10–14 days post-operatively, 5 patients (20.5%) had a positive result. Thirteen (41.9%) patients had recurrent or treatment-refractory MB; three (23.1%) of these patients were infants. At 5 years, the recurrence-free survival was 55.7% (95% CI: 37.5%–73.7%) and the OS was 62.8% (95% CI; 45.0%–80.5%).
Table 1: Patient Demographics (n = 31)
WT = wild type; G4 = Group 4.
Progressive or Recurrent Disease Carries Significant Mortality Especially in the Infant Group
The median time to either disease progression or recurrence was 14.6 months from the first presentation (Table 2). Thirteen of 31 (41.9%) children were found to have metastatic disease at presentation. Furthermore, children presenting with metastatic disease had a higher likelihood of recurrence (6 of 13 (46.2%) vs. 7 of 18 (38.9%)). Recurrence in infants occurred earlier than in non-infants (median 3.1 months (28.0 months) vs. 15.1 months (35.0 months) (Figure 1A), and infants with recurrence had a shorter median follow-up (i.e., 7.2 months (45.6 months)) compared to non-infants (i.e., 39.6 months (69.5 months)), as the tumor in these patients tended to return or progress aggressively and was more likely to be palliated. Patients who experienced recurrence and were alive at the time of writing had a median follow-up of 58.8 months (38.4 months) since admission, and of these children, two out of four (50.0%) had metastasis at presentation. Comparatively, patients who experienced recurrence and were deceased had a median follow-up of 25.2 months (53 months) since admission, and four out of nine (44.4%) had metastasis at presentation. With respect to the location of recurrence, among the 13 patients who had progression or recurrence of their tumor, only 3 (23.1%) experienced local disease progression/recurrence while the remaining 10 patients (76.9%) recurred with disseminated leptomeningeal spread (Table 3). Median time to recurrence was 15.1 months (7 months) for patients with local recurrence, compared to 13.5 months (35 months) for patients with leptomeningeal spread (Figure 1B). While none of the patients with local recurrence had metastasis at presentation, 6 out of 10 (60.0%) patients with leptomeningeal spread had metastasis at the time of the first presentation. Although all the children within the local recurrence group were alive with stable disease at the time of writing, none had reached the 5 years of follow-up (range: 0.7–2.1 years). In the group experiencing leptomeningeal recurrence, however, two (20%) were alive more than 5 years after an initial diagnosis with three (33.3%) alive with stable disease and a median follow-up of 5.4 years (6.4 years) at the time of writing. Median follow-up after recurrence was 10.2 months (23 months) in the local group, compared to 14.5 months (75 months) in the leptomeningeal group.
Table 2: Age stratification into infant and non-infant populations and clinical characteristics
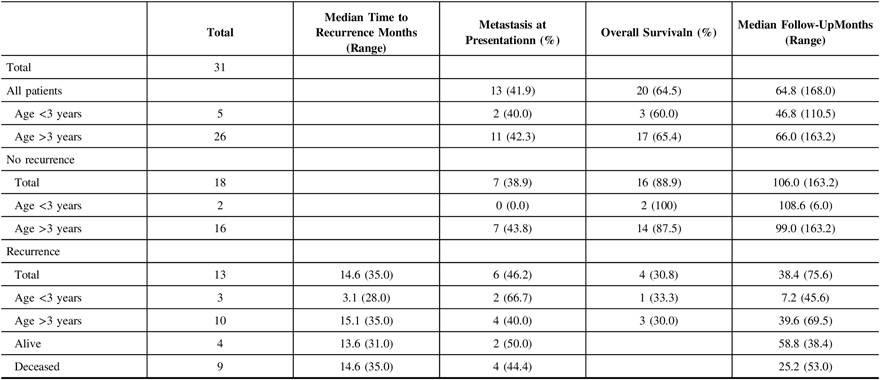
Table 3: Medulloblastoma recurrence pattern
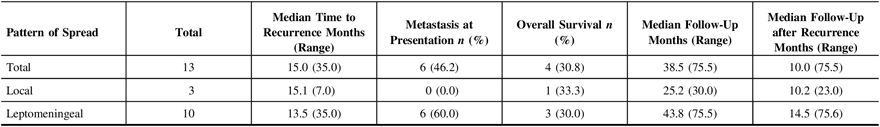
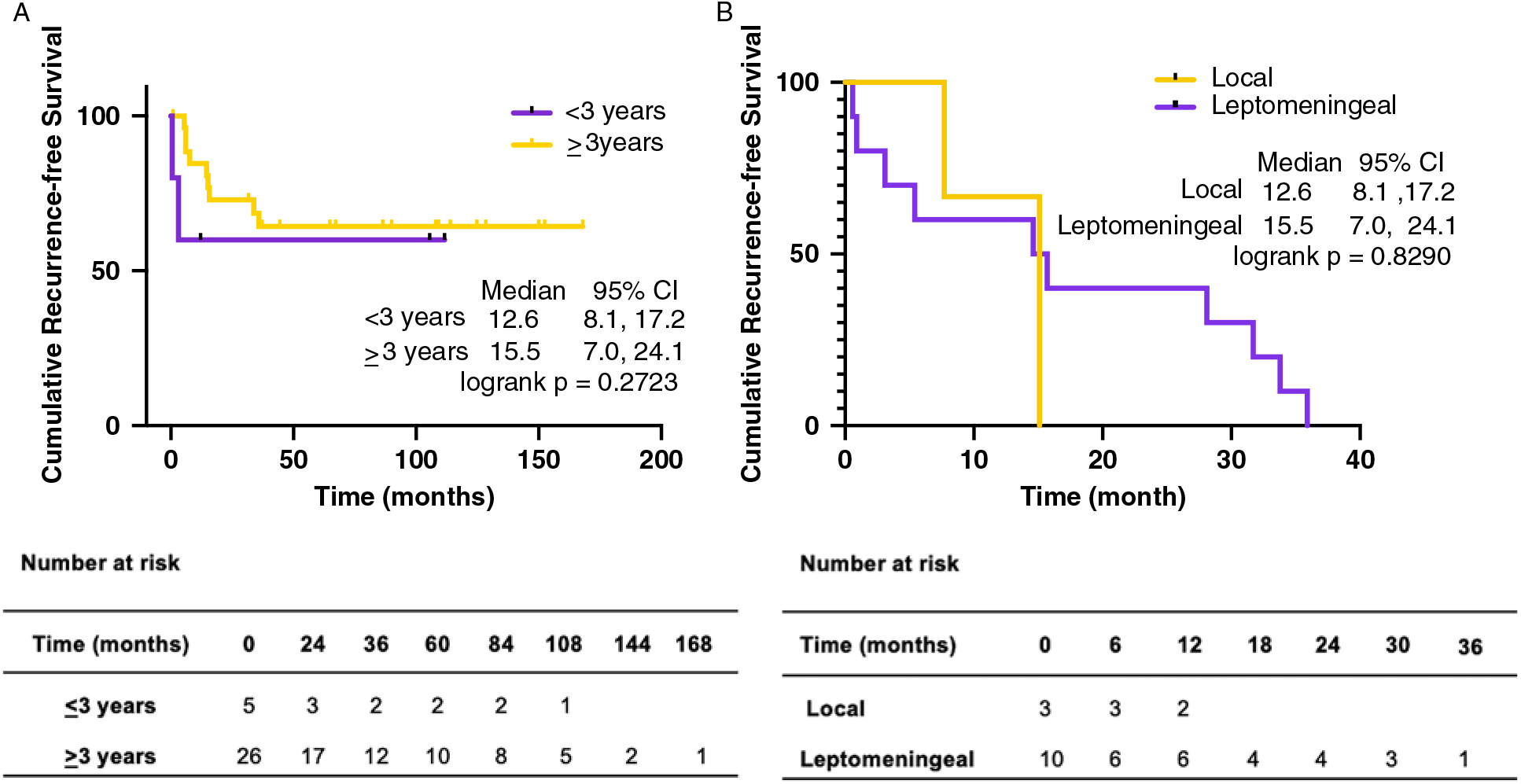
Figure 1: Cumulative recurrence-free survival by age and pattern of recurrence. (A) Cumulative recurrence-free survival by age and (B) cumulative recurrence-free survival by pattern of recurrence with associated at risk tables.
Post-Progression or Disease Recurrence Management
In 3 of the 13 (23.1%) patients experiencing recurrent MB the disease had significantly progressed, and patients were palliated (Table 4), with a median time to recurrence of 3.0 months (4 months). Two of these children had metastasis at presentation, with a median follow-up of 3.0 months since admission (4.8 months); median follow-up since recurrence was 0.1 months (1.2 months). The remaining 10 (76.9%) children underwent some form of salvage therapy that was delivered in isolation or in combination with the other modalities. The treatments provided included surgical re-resection, radiation (CSI, whole-brain irradiation, or focal stereotactic irradiation delivered via a robotic arm called CyberKnife®), drugs such as imatinib, etoposide, vinblastine, temsirolimus, tipifarnib, temozolamide, and irinotecan, either in isolation or in varying combinations. Among patients who received salvage therapy, follow-up since presentation ranged from 7.2 to 76.8 months, while follow-up since recurrence ranged from 0.4 to 75.8 months. Of the 10 patients who received salvage therapy, 4 (40.0%) were alive at the time of writing (Table 5). Three out of the four children experienced leptomeningeal dissemination.
Table 4: Salvage therapy received in patients with recurrence (n = 13)

CSI = craniospinal irradiation.
Table 5: Description of treatment course for survivors receiving salvage therapy (n = 4)
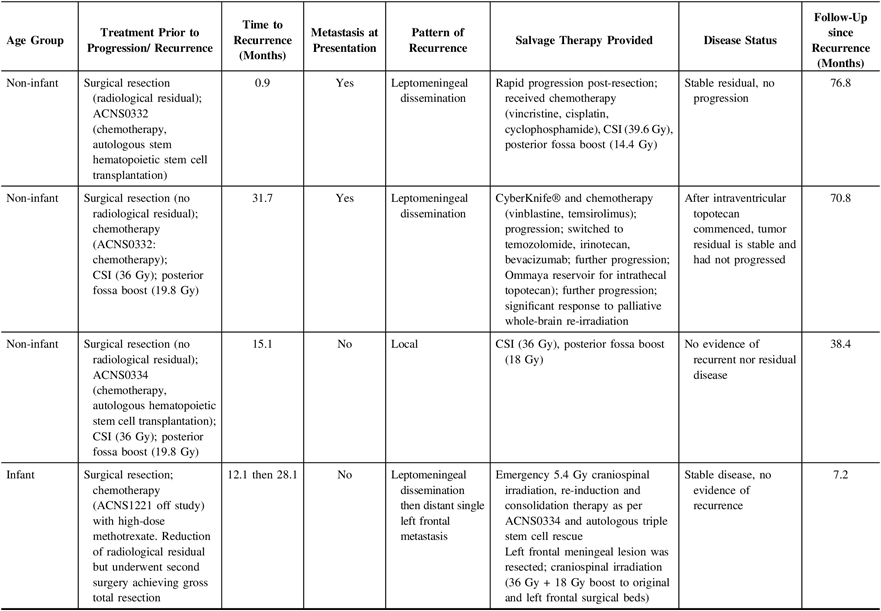
CSI = craniospinal irradiation.
Of note, there were two patients who did not experience recurrence but died secondary to other medical complications. One patient died from respiratory compromise in hospital, and the second patient from neutropenic colitis, acute respiratory distress syndrome (ARDS), and subsequent cardiorespiratory arrest. Both were stratified as high-risk MB patients, underwent GTR, and were subsequently treated with chemotherapy (vincristine, cisplatin, cyclophosphamide, etoposide) in addition to CSI with a posterior fossa boost.
Molecular Subgrouping and Clinical Outcomes
Molecular subgrouping was available for eight patients (Table 6). Two were Wnt MB, and neither experienced recurrence and were alive with stable disease for a mean of 3.4 years (3.1–3.7). Two patients’ tumors had a significant Shh pathway activation with recurrent disease experienced by both patients. One was an infant with a desmoplastic nodular histological appearance with p53 wild type (WT) immunoreactivity, and the other anaplastic histological appearance and also p53 WT. The remaining four patients were G4 MB. One G4 MB had an STR, metastatic at presentation and risk stratified into high-risk upfront therapy and disease-free 5.6 years since presentation at the time of writing. Three of the four G4 MB were metastatic at presentation and risk stratified into high-risk therapy with two recurring 33.8 and 15.1 months after presentation. One experienced progressive disease despite re-resection, focal re-irradiation more than 2 years after their first exposure to CSI in addition to temozolamide and etoposide and therefore palliated. The second patient received focal irradiation via CyberKnife® but was palliated due to complications of the adjuvant therapy. The standard risk G4 MB patient experienced recurrence 15.1 months after GTR, had CSI for salvage therapy, and was alive 2 years with stable disease since the recurrence.
Table 6: Clinical course and outcomes of children with molecular subgrouping data (n = 8)

WT = wild type; Wnt = Wingless; Shh = Sonic hedgehog; G4 = Group 4 molecular subgroups; CSI = craniospinal irradiation.
Discussion
This retrospective single center study highlights the heterogeneity of salvage treatments provided to children with treatment-refractory or recurrent MB between 2003 and 2015.
Timing and Pattern of Recurrence
Thirty-one children with a diagnosis of MB were treated in our institution over a 13-year period. Similar to other pediatric MB cohorts, our cohort consisted of infants and children with a predilection of disease in males and lower incidence in children less than 3 years of age.23, Reference Kopecky, Khan, Pan, Drachtman and Parikh29–Reference Rickert and Paulus31 Thirteen of 31 children experienced treatment-refractory disease, local recurrence, distant metastases, or florid leptomeningeal dissemination. We included children who had at least 2 years of follow-up data because it is generally believed that once the child has completed the treatment course, and his or her 2-year follow-up imaging is free of disease, the child is generally considered to have a more favorable prognosis compared to those with imaging suggestive of progressive disease.Reference Koschmann, Bloom, Upadhyaya, Geyer and Leary32, Reference Dhellemmes, Demaille, Lejeune, Baranzelli, Combelles and Torrealba33 However, in a recent large multicenter study that analyzed the survival of children with recurrent MB, it was noted that risk-based therapy results in relatively stable 5-year PFS and OS rates but with tumor recurrence observed in a significant number of children out with the 5-year interval.Reference Johnston, Keene and Strother34 Similarly, in our cohort there were three children who had recurrence of their disease out with the 2-year window. Comparable to Koschmann et al.’s cohort who had a median time to recurrence of 18 months,Reference Rickert and Paulus31 our cohort had a median time to recurrence of 15.0 months (35.0 months) from the date of admission, and as expected the children requiring salvage therapy were more likely to have presented with evidence of radiological metastasis. As previously described23 phenotypically more aggressive disease was also observed in our infant cohort as compared to non-infants with earlier recurrence (3.1 vs. 15.1 months) and shorter survival (3.0 vs. 33.6 months). Given the small numbers of patients with molecular subgrouping data in our cohort, we observed recurrence in 60% (i.e., 3 of 5) of the subgrouped within the 2-year interval (Table 6).
Although none of the children in our locally recurring MB cohort presented with metastatic disease, this group had a comparable 5-year OS to the leptomeningeal recurrence cohort suggesting that recurrent disease is a poor prognostic indicator irrespective of its pattern of disease. Ramaswamy et al. were the first to describe the timing and pattern of MB recurrence in molecularly subgrouped MB, observing G4 MB tumors to have the longest recurrence-free interval and post-recurrence survival.Reference Cho, Tsherniak and Tamayo19 G3 MB tumors were observed to experience the opposite clinical course (i.e., shortest recurrence-free and post-recurrence survival interval). In contrast to Ramaswamy et al.’s description of Shh tumors more commonly experiencing local recurrence with metastatic recurrence occurring more commonly in G3 or G4 MB tumours,Reference Cho, Tsherniak and Tamayo19 in our small cohort of subgrouped recurrent MB cohort, we observed the opposite pattern with both our Shh subgrouped patients recurring with florid disseminated disease and the G4 MB recurring locally.
Salvage Therapy Provided
The salvage therapy provided to our cohort of children was largely dependent on the upfront treatment administered after the initial resection. At disease progression or recurrence, the armamentarium for salvage therapy includes second surgery, chemotherapy, and radiotherapy. Although Johnston et al. demonstrated that once MB has recurred, the probability of regaining tumor control is poor,Reference Koschmann, Bloom, Upadhyaya, Geyer and Leary32 and this observation may be more speaking to the lack of salvage therapy available to the child because of the treatment that patient has already received, poor tolerability of the salvage therapy, the child’s neurological status, and bone marrow and end-organ function. In three patients experiencing recurrence/disease progression (23%), the disease progressed beyond that would have been amenable to salvage therapy and therefore managed expectantly on a palliative care pathway. In the remaining 10 children (77%) however, a number of salvage therapy protocols were provided based on available clinical trials with 6 of the children in our cohort (i.e., 6/10 recurrent/treatment-refractory MB receiving salvage therapy, 60%) succumbing to recurrent/progressive disease. Their clinical course is comparable to other large pediatric MB datasets, where they were more likely to be metastatic at presentation, with a poor OS.Reference Ramaswamy, Remke and Bouffet1, Reference Pietsch, Schmidt and Remke35–Reference Chan, Teo, Seow and Tan37 Of interest, half of the surviving recurrent children continue to survive their disease despite their metastatic status at presentation (Table 5).
The location of disease recurrent was insignificant for OS in our cohort. Of the three children who presented with local disease progression/recurrence, only one was alive at the time of writing. The first child who died of local progression showed extension of the recurrent lesion from the original tumor bed, crossing the midline into both cerebellar peduncles and the midbrain. He was administered etoposide but was palliated and died shortly thereafter. The second child succumbed to the disease after Cyberknife® focal irradiation treatment was complicated by pancytopenia, infection, encephalitis, status epilepticus, and disseminated intravascular coagulation. Both of these children had an intraoperative surgical impression of GTR, and neither had a radiological residual tumor detected on post-operative MRI. The third child, currently surviving a local recurrence, was initially treated under the standard risk regimen and was alive at the time of writing, 1.5 years after receiving CSI as salvage therapy. This case in particular highlights the role of re-irradiation as a salvage therapy to be considered, particularly for the relapsed standard risk patients.Reference Wetmore, Herington, Lin, Onar-Thomas, Gajjar and Merchant38
Of the children recurring with leptomeningeal dissemination, three were palliated (i.e., two infants, one non-infant) due to the extensive form of disease. Of the remaining seven children, one was an infant with desmoplastic nodular histology and Shh subgrouping recurred with leptomeningeal dissemination (Case 3). Of the six non-infants experiencing leptomeningeal disseminated recurrences, two were alive at the time of writing. One child recurred and progressed through multiple salvage strategies with disease control with whole brain re-irradiation (Case 2). The remaining non-infant patient experienced a rapid progression prior to CSI treatment, and disease control was achieved with urgent CSI, vincristine, cisplatin, and cyclophosphamide (Case 1). Both these non-infant children were diagnosed with an anaplastic histopathological subtype with the former patient’s tumor subgrouped as a Shh. The latter patient’s subgroup was yet to be determined.
Treatment of Recurrent Pediatric MB in the Molecular Era
When molecular subgrouping for pediatric MB became the standard of care, the opportunities to understand the biological mechanism of disease and consequently options for post-resection adjuvant therapy experienced a significant development. Furthermore, a deeper insight into which MB subgroups are associated with a favorable prognosis and therefore can be considered for de-escalation therapy became a rationalizable option. While the patient management of recurrent MB historically focused on the quality of the remaining life of the child rather than curative strategies, informed by the molecular data from childhood MB tumor samples, and in collaboration with drug companies, pediatric neurooncologists and the scientific community have been able to explore the possibility of individualized targeted therapy.Reference Gottardo, Hansford and McGlade39 The recognition of the genetic subgroups of pediatric MBReference Beutler, Avoledo and Reubi17 and its incorporation into the 2016 WHO Classification of tumors of the central nervous systemReference Louis, Perry and Reifenberger40 have led to a change in clinical management of our locally treated patients where all samples are now sent to a CLIA-certified laboratory for molecular subgrouping. Although subgroups are believed to remain stable at recurrence.Reference Cho, Tsherniak and Tamayo19, Reference Wang, Dubuc and Ramaswamy41 Hill et al. identified new mutations that occur in all subgroups (e.g., MYC/MYCN amplification and p53 mutation) at relapse following the standard upfront therapy,Reference Hill, Kuijper and Lindsey42 suggesting a divergence in genetic profiles of MB tumors at recurrence as Morrissy et al. observed.Reference Morrissy, Garzia and Shih43 Not unexpectedly, however, the only survivors after salvage therapy in Hill et al.’s cohort were of the Wnt subtype. In our surviving recurrent pediatric MB cohort, however, two patient tumors were subgrouped as Shh without p53 mutation, one G4 MB of classic histology and the remaining of unknown molecular subgrouping (Table 6).
Case 1: A Very Young Metastatic G4 MB Successfully Salvaged with CSI and Chemotherapy
One of the four patient survivors was molecularly subgrouped as G4 MB with metastasis at presentation but, given their young age, underwent a radiation sparing protocol only to progress soon after the completion of consolidation therapy and autologous stem cell rescue. According to Ramaswamy et al., molecular risk stratification for this patient was very high-risk secondary to the G4 MB subgrouping with metastasis at presentation.Reference Ramaswamy, Remke and Bouffet1 As expected, despite upfront radiation sparing therapy, this young child recurred with leptomeningeal dissemination. At the time of recurrence, the child was old enough to receive full dose CSI with a posterior fossa boost and chemotherapy and has had stable disease for over 5 years.
Case 2: Non-Infant Shh Successfully Salvaged with Multiple Agents and Modalities
The first of two Shh subgrouped tumor patients was diagnosed just before their ninth birthday. No germline mutations were identified, and they received upfront therapy according to high-risk group stratification.Reference Polkinghorn and Tarbell2 They were salvaged with focal irradiation using CyberKnife® of the local recurrence in the tumor bed and adjuvant chemotherapy. Having progressed mainly intraventricularly subsequent salvage therapy involved an Ommaya reservoir insertion for intrathecal topotecan. The intraventricular component remained stable until a follow-up MRI just under a year later revealed florid recurrence. At this time, no curative option was available, but palliative whole-brain radiation in the form of 20 Gy in five fractions was offered to prolong survival. The intracranial tumors had a remarkable response, and the patient is currently monitored expectantly with the radiation oncologists’ recommendations that if there is limited growth of <2 lesions, focal irradiation with CyberKnife® would be an option. This case illustrates the important role of re-irradiation in pediatric MB, if safe to deliver, to provide long-term tumor control. A case series of patients from St. Jude’s with recurrent MB treated with or without re-irradiation observed a striking benefit of re-irradiation in the standard risk group with a 10-year OS of 45% in those who were irradiated and 0% for the patients who did not receive re-irradiation (p = 0.036).Reference Ellison, Kocak and Dalton36 Similar 10-year OS benefit was observed in the high-risk group (p = 0.003) with the caveat that the analysis was limited by the small number of patients in this cohort. Fortunately, this patient’s very aggressive tumor recurrence responded to whole-brain re-irradiation, provided as a palliative therapy, with stable disease and unexpected functional recovery for the last 6 months at the time of writing.
Case 3: Desmoplastic Nodular Infant Shh MB with Recurrence and Effective Salvage
An infant diagnosed just before his second birthday presented with a 1-month history of failure to thrive and persistent vomiting. Imaging demonstrated a large posterior fossa mass with associated severe hydrocephalus but no evidence of metastatic disease (Figure 2A). The intraoperative impression was GTR, but the post-operative MRI identified a 1.9 cm residual (Figure 2B) and the histopathological diagnosis of desmoplastic nodular MB. Given the patient’s age, histology suggestive of better prognostic type of MB, the molecular subgroup suggestive of Shh pathway activation, and the HIT-SKK European MB trialsReference Friedrich, von Bueren and von Hoff44 suggesting Shh MB making up the majority of desmoplastic nodular MB, have a much better prognosis, the traditional intensive chemotherapy with autologous stem cell transplant was thought to be too toxic, and therefore the patient underwent an intermediate dose chemotherapy protocol (ACNS1221). Although the residual reduced to half the size (Figure 2C) after the initial three cycles of induction chemotherapy (i.e., vincristine, high-dose methotrexate, carboplatin, etoposide, cyclophosphamide, MESNA), following careful multidisciplinary team consideration, the patient underwent a second-look surgery to resect the residual tumor, achieving GTR. When the residual disease pathology confirmed MB, the next two consolidation cycles of chemotherapy (i.e., carboplatin, etoposide, and MESNA) resumed. Three months after the completion of treatment, the patient returned to the ED with vomiting, and was found to be bradycardic and hypertensive. An immediate MRI scan revealed significant hydrocephalus secondary to extensive recurrent disease (Figure 2D). external ventricular drain (EVD) was inserted, and the patient was again provided a lower dose of chemotherapy (i.e., temozolamide and irinotecan) for 5 days. As no progression of disease was noted in the interval MRI the child was treated with three fractions of whole-brain radiation (1.8 Gy each) in order to control the tumor while the chemotherapy commenced. The induction chemotherapy was started as per ACNS0334 with high-dose methotrexate to provide intensive re-induction for patients who demonstrate good disease control while on chemotherapy. The re-induction therapy yielded a significant reduction in overall disease burden with the complete resolution of leptomeningeal disease and supratentorial enhancement, only leaving a small nodular enhancement in the posterior fossa. This remarkable response to chemotherapy justified the use of consolidation therapy and autologous stem cell rescue. The consolidation therapy (i.e., triple platinum carboplatin and thiopenta) treated the residual nodular disease, and the patient remained in remission for the next 28.1 months when a routine follow-up MRI identified a left frontal enhancing lesion (Figure 2E). Suspicious of distant metastasis, surgical resection was undertaken and after confirmation of MB histology, the patient was treated with adjuvant CSI (36 Gy) with a further boost to the original and the left frontal metastatic tumor beds (18 Gy). The child has been alive for 7.2 months with stable disease since the identification of the distant metastatic recurrence. Interestingly, genetic testing revealed a germline SUFU mutation in the absence of any other genetic or phenotypic abnormalities (e.g., no PTCH-1 mutation, cutaneous cancer lesions, or jaw cysts) previously described as a predisposing mutation in the development of MB.Reference Taylor, Liu and Raffel45 Clinical trials investigating the role of smoothened (SMO) inhibitors in relapsed pediatric Shh subgrouped MB exist; however, the early trials failed to show efficacy likely because of their inclusion of children with genetic mutations that are downstream of SMO (e.g., SUFU).Reference Kieran, Chisholm and Casanova46

Figure 2: Radiological images of a local and distant recurrent infant desmoplastic nodular medulloblastoma. (A) At presentation, a heterogeneously enhancing large posterior fossa lesion with associated hydrocephalus, (B) Post-operative 1.8 mm residual at the superior border of fourth ventricle, (C) 7 months after adjuvant induction chemotherapy (ACNS1221) showing reduction in size of the residual, (D) 3 months post-completion of consolidation therapy (ACNS1221) showing rapid recurrence with diffuse leptomeningeal disease, And (E) 21 months post-completion of emergency 5.4 Gy craniospinal irradiation and re-induction and consolidation therapy (ACNS0334) showing left frontal avidly enhancing leptomeningeal lesion.
A similar recent report of effective salvage of two children with desmoplastic MB recurring on the ACNS1221 trial was published by Graham et al.Reference Graham, Conley, Finlay and AbdelBack47 The first child was 3 years at the time of diagnosis and recurred 3 months post-therapy undergoing a five-drug treatment regimen (i.e., vincristine, cisplatin, etoposide, cyclophosphamide, and high-dose methotrexate) with autologous tandem transplants and proton CSI (23.4 Gy) with a boost to the posterior fossa (54 Gy) and the spine (45 Gy) for salvage therapy. The second child was a 4-year-old male with Gorlin’s syndrome and Trisomy 21 experiencing a local recurrence 9 months after undergoing therapy and was successfully salvaged with three cycles of chemotherapy (i.e., carboplatin and thiopental), and autologous tandem stem cell support without the need for radiation therapy. Although Shh MB is the most common molecular subtype making up the desmoplastic MB histopathological subtype, it is now clear that there are biological differences responsible for the difference in clinical response to therapy that is observed even within the desmoplastic nodular MB subtype. Relevant to our detailed infant desmoplastic nodular MB case, a recent retrospective study of 22 patients from 17 families with a germline SUFU mutation reported a worse 5-year PFS and OS of 42% and 66% as compared to Shh MB without SUFU mutationsReference Guerrini-Rousseau, Dufour and Varlet48 to explain the unfavorable clinical course experienced by this particular infant. In addition, two recent risk-adapted therapy trials, SJC07 and ACNS1221, found that risk-adapted therapy did not significantly improve event-free survival prompting the need for a deeper understanding of the biology of pediatric MB to guide an appropriate individualized upfront therapy.Reference Schwalbe, Lindsey and Nakjang24, Reference Lafay-Cousin, Bouffet, Onar-Thomas, Billups, Hawkins, Eberhart, Horbinski, Robinson, Strother, Heier, Souweidane, Fouladi and Gajjar49
In summary, the overall outcome survival of children experiencing treatment-refractory or recurrent MB is poor. Furthermore, although the salvage strategy for these children appears to have been individualized, further advancement in our understanding of the implications of molecular subgrouping will increasingly facilitate and guide the use of not only upfront molecular therapy but also has the potential to change the landscape of salvage therapy options for treatment-resistant or recurrent pediatric MB.
Strengths and Limitations
This study had several strengths. Missing data were minimal, given the close monitoring and documentation of the children within the pediatric neurooncology service, in addition to the details added by the members of the PBTSG directly involved in the child’s care. In addition, as this is a rare diagnosis, the long duration of participant inclusion reflects the systematic tracking of these children in our institution.
This study is limited by all the factors inherent in conducting a retrospective analysis. The small cohort and heterogeneity of the tumor subtypes of recurrent MB patients limit recommendations with regard to the efficacy of the salvage therapies provided. Although molecular-level data have identified which MB subgroups are at greater risk, prior to 2016 many institutions did not routinely integrate subgrouping into their histopathological diagnosis. In order to facilitate the generation of statements regarding the outcomes of the changes in practice, historical data with sufficient granularity are required. We feel that we have provided this level of detail for our single center cohort and that a multicenter cohort study would have the power to better describe and make recommendations from international experiences treating recurrent pediatric MB within the molecular era.
Conclusion
Recurrent pediatric MB in our single center cohort carried a poor prognosis despite the administration of salvage therapy. Although there is standardization of the upfront treatment for these children, we observed great heterogeneity in the treatment of patients experiencing recurrence secondary to either the upfront therapy they had received or tolerability of the therapy proposed. A greater understanding of the biology of recurrent MB as well as systematic data capture in multicenter studies has the potential to guide salvage therapy.
Acknowledgements
The authors would like to acknowledge all the individuals who were vital in the production of the dataset that made the analysis for this study possible.
Disclosures
None of the authors have any conflicts of interest to disclose.
Author Contributions
MKS, AF, SKS, MCS, JD, MCA, BY, and OA conceptualized the project. MKS, AS, AM, AMP, and AAA acquired data. MKS and DB coordinated and performed experiments for NanoString subgrouping data analysis in the laboratory of SKS. FF, NA, MKS, AAA, and AF analyzed and interpreted the data. MKS and AAA wrote the manuscript with revisions contributed by AF, MCS, SKS, AAA, and DB. AF and SKS provided guidance related to pediatric neurooncology clinical and pre-clinical, trials respectively. AF and SKS supervised the study. All authors reviewed the results and commented on the manuscript.










