Introduction
Background/Rationale
Primary progressive aphasiaReference Gorno-Tempini, Hillis and Weintraub1 is a neurodegenerative disorder whose prominent feature is language impairment,Reference Mesulam2 as opposed to early episodic memory or motor deficits that are predominant in other dementias. The semantic variant of primary progressive aphasia (svPPA), also called semantic dementia, is one of its three variants, alongside progressive non-fluent aphasia and logopenic progressive aphasia. These aphasias are forms or phenotypes of frontotemporal lobar degeneration,Reference Riedl, Mackenzie and Förstl3, Reference Mesulam, Wicklund and Johnson4 a family of diseases that represent 10% of all dementias in adults <65 years old.Reference Fiest, Jetté and Roberts5, Reference Hogan, Jetté and Fiest6 It has been often reported that logopenic PPA is most often Alzheimer’s disease, and not a frontotemporal dementia per se.Reference Ahmed, de Jager and Haigh7–Reference Teichmann, Kas and Boutet9
Clinically, features of svPPA include impairment in confrontation naming and single-word comprehension and may include surface dyslexia or dysgraphia, while repetition and speech production is preserved.Reference Routhier, Gravel-Laflamme and Macoir10 Pathologically, svPPA appears to be associated with ubiquitin and TDP-43.Reference Mesulam, Wicklund and Johnson4, Reference Hodges, Davies and Xuereb11–Reference Nestor, Balan and Cheow13 Anatomically, changes in cortical grey matter (GM) have been well studied in svPPA, to the extent that imaging can be used as a supportive criterion for the diagnosis. These findings include the presence, on magnetic resonance imaging (MRI), of cortical atrophy located predominantly in the anterior temporal lobes, bilaterally but more severely in the left hemisphere, especially in its ventral and lateral regions.Reference Gorno-Tempini, Dronkers and Rankin14–Reference Collins, Montal and Hochberg19 In studies involving presymptomatic subjects, focusing on correlating genetics and imaging, researchers have found that GRN mutations are associated with GM changes in the striatum, insula and with early temporal and parietal atrophy, while MAPT mutations are associated mainly with temporal atrophy, particularly in its anteromedial portion.Reference Rohrer, Nicholas and Cash20–Reference Whitwell, Weigand and Boeve23
Comparatively, the extent of white matter (WM) damage in svPPA has been much less studied, in part due to the difficulty in recruiting large patient groups. Only a few reportsReference Galantucci, Tartaglia and Wilson24–Reference Mahoney, Malone and Ridgway27 have addressed this topic (largest N = 10). They all made use of diffusion tensor imaging (DTI), a technique that allows the study of WM fibre tracts in MRI, and from which one can extract maps of fractional anisotropy (FA), a scalar value indicating the directionality of diffusion in axons, itself a proxy of microstructural damage in the brain.Reference Basser and Pierpaoli28 These studies reported lower FA mostly lateralised in the left hemisphereReference Vandenberghe29 and affecting the postero-inferior longitudinal, uncinate and posterior cingulate fascicule,Reference Galantucci, Tartaglia and Wilson24–Reference Mahoney, Malone and Ridgway27 in opposition to non-fluent primary progressive aphasia that appears to affect the left intrafrontal and frontostriatal fascicule.Reference Mandelli, Caverzasi and Binney30
Furthermore, all of these cited WM studies but one used a region-of-interest rather than a tract-based statistical (TBSS)Reference Smith, Jenkinson and Johansen-Berg31 approach to analyse FA maps. The latter is a whole-brain skeleton-based technique that is more sensitive than the ROI voxel-based technique, as demonstrated in a simulation study;Reference Ball, Boardman and Arichi32 and hence that should provide a better resolution of WM damage in the brains of svPPA patients.
Objectives
We designed this study to increase our current knowledge of concurrent WM and GM damage in svPPA in a different study group. Our objectives were to localise and quantify cerebral GM and especially WM differences in patients with svPPA, compared with cognitively healthy controls, through automated cerebral segmentation and tract-based statistics of fractional anisotropy.
Method
Ethics
The ethics committee of the Institut universitaire en santé mentale de Québec approved the study (project #300-2012). Informed and free consent was obtained from each participant.
Study Design
We used a between-group experimental design, in which we compared patients with a diagnosis of svPPA with cognitively and neurologically healthy, age-matched control subjects. All participants were native French speakers from Quebec, Canada.
Setting and Participants
Patients – Ten patients who fulfilled current svPPA clinical criteriaReference Gorno-Tempini, Hillis and Weintraub1 and were being followed at Clinique Interdisciplinaire de Mémoire (CHU de Québec, Quebec City, Canada) between August 2013 and August 2014 were included in this study. These patients all had a diagnosis of svPPA confirmed by a neurologist, without family histories indicative of a possible genetic risk. Additionally, a comprehensive neuropsychological battery was administered to all participants. The most salient results of this battery included:
Mild to moderate word production and anomia difficulties measured with a picture-naming task (Boston Naming Test)Reference Kaplan, Goodglass and Weintraub33 and phonological, semantic and free fluency tasks (MEC Protocol);Reference Joanette, Ska and Coté34
Impaired verbal and non-verbal single-word comprehension, assessed with visual and oral word–picture matching tasks (BECLA battery)Reference Macoir, Gauthier and Jean35 and semantic association of pictures (pyramids and palm trees test);Reference Howard and Patterson36
Spared repetition (TEFREP)Reference Bourgeois-Marcotte, Wilson and Forest37 and absence of apraxia of speech (PENO).Reference Joanette, Ska and Belleville38
Some of the patients also had impaired object recognition (object decision and object matching subtests, BORB battery)Reference Riddoch and Humphreys39 with or without surface dyslexia (regular and irregular word and pseudoword reading test),Reference Wilson, Joubert and Ferré40 while others presented with surface dyslexia with unimpaired object recognition. They all were free of noticeable deficit on tests tapping other cognitive domains such as non-verbal episodic memory, visuospatial abilities (Rey-Osterrieth Complex Figure Test)Reference Meyers and Lange41 and working memory (forward and backward digit SPANs Wechsler Memory Scale IV).Reference Wechsler42
Controls – Cognitively healthy controls were recruited among patients’ proxies and members of the community and were matched by age, gender, education and manual dominance to the svPPA patients. They were screened via telephone for inclusion criteria by means of a structured questionnaire. Then they went through the same neuropsychological battery as did the patients.
Neither patients nor controls had histories of either moderate or severe traumatic brain injury, cerebrovascular disease or intracranial surgery, encephalitis or meningitis. They had no history of a significant psychiatric syndrome, alcoholism or drug addiction or unstable medical or metabolic condition (e.g., uncontrolled diabetes, vitamin B12 or folic acid deficiency, hypothyroidism), nor any history of learning or reading difficulties. They were also screened for MRI compatibility by means of another questionnaire.
Data Sources and Measures
Above and beyond the tests previously mentioned, the demographic and clinical data included the presence or absence of semantic dementia, age, gender, education (in years), manual dominance, and global cognition assessed via the Montreal Cognitive Assessment Test (MoCA)Reference Nasreddine, Phillips and Bédirian43 and Mini-Mental State Examination (MMSE).Reference Folstein, Folstein and McHugh44 Since both groups of participants were matched by age, gender, education and manual dominance, we did not make further sensitivity analyses. It would have been unlikely that the 10:9 ratio of patients and controls affected the volumetric or FA data.
MRIs were acquired within 4 weeks of the clinical assessment using the Canadian Dementia Imaging ProtocolReference Duchesne, Chouinard and Potvin45 (www.cdip-pcid.ca) on a 3-T magnetic resonance scanner (Philipps Medical Systems, Best, The Netherlands) at the IRM Québec radiology clinic in Quebec City. Specifically, the protocol included:
An isotropic, 1 mm3 3D T1-weighted sequence (TR = 7.3 ms; TE = 3.3 ms; flip angle = 9 degrees); and
A 60-direction, 2 mm3 isotropic diffusion imaging sequence (b-value = 1000).
For cerebral WM data, fractional anisotropy maps and tract-based statistics were obtained following these steps. First, we converted our native data from DICOM files to the NIFTI format using MRIcron (http://www.mricro.com/mricron). Then, we used FMRIB’s Diffusion ToolboxReference Smith, Jenkinson and Woolrich46 to create an FA image from the DTI data of each subject. This included eddy current correction, brain masking and diffusion tensor model fitting. Afterwards, we used the FMRIB Software Library 5.0.6Reference Woolrich, Jbabdi and Patenaude47 to perform voxel-wise analyses, following the TBSS approach.Reference Smith, Jenkinson and Johansen-Berg31 There were five steps to this process: (1) pre-processing; (2) nonlinear alignment of all FA images to a standard-space image (FMRIB58_FA); (3) creation of the mean FA image and generation of its skeleton; (4) projection of every pre-aligned FA image on the study-specific template skeleton; and (5) generation of voxel-wise statistics with the Randomise tool, using the Threshold-Free Cluster Enhancement. The final output was a brain map in which we could identify voxels that were statistically different in FA between patients and controls. We used the Atlasquery tool to localise these voxels and provide numerical FA values for each subject in each of the 48 regions of interest (ROIs) identified in the ICBM-DTI-81 WM labels atlas of the Laboratory of Brain Anatomical MRI at Johns Hopkins University.Reference Oishi, Faria and Jiang48
For GM analysis, we used an automated segmentation tool (FreeSurfer)Reference Dale, Fischl and Sereno49, Reference Desikan, Ségonne and Fischl50 to segment the 3D T1-weighted image for all subjects, producing surfaces (mm), thicknesses (mm2) and volumes (mm3) for the whole brain and 44 different cortical and subcortical structures according to their atlases (DKT40 and ASEGReference Fischl, Salat and Busa51).
Statistical Methods
For all demographic, clinical and imaging data, we opted for non-parametric Wilcoxon rank-sum test to compare cases with controls, given the size of our sample and the robustness of the test. We used a significance level of p-value = 0.05, correcting for multiple comparisons using the false discovery rate (FDR) method.
Reporting
This study follows the recommendation of the STROBE Statement.Reference von Elm, Altman and Egger52
Results
Demographic and Clinical Data
Nineteen participants (10 svPPA and 9 controls) were recruited in this study. Every subject that was screened happened to be eligible for the study. Analysis of demographic data showed no significant difference between groups in age, gender, education or manual dominance. Clinical data showed significantly lower results for svPPA patients at both MoCA and MMSE tests, which was expected (see Table 1).
Table 1: Demographic and clinical data for svPPA patients and controls
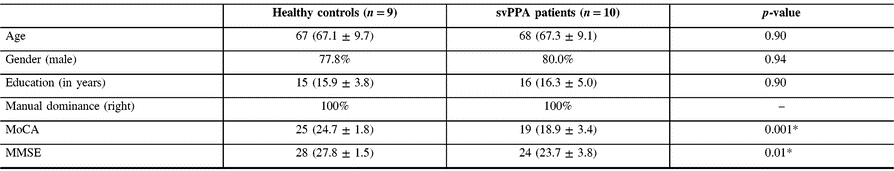
svPPA = semantic variant of primary progressive aphasia; MoCA = Montreal Cognitive Assessment; MMSE = Mini-Mental State Examination.
* Statistically significant.
Results are presented as median (mean ± standard deviation).
WM Analysis
Volumetric data showed that both groups had similar total WM volumes. Qualitative assessment of diffusion data demonstrated multiple WM regions where svPPA patients had significantly lower FA values, slightly more severely in the left hemisphere and temporal lobe. Statistical analysis of numerical FA values resulted in 16 out of 48 tract regions with significantly lower FA values in svPPA patients than in healthy controls, after FDR correction. The most significantly impaired regions were the right hippocampus and uncinate fasciculus tract regions, and the left external capsule and superior longitudinal fasciculus (see Table 2 and Figure 1).
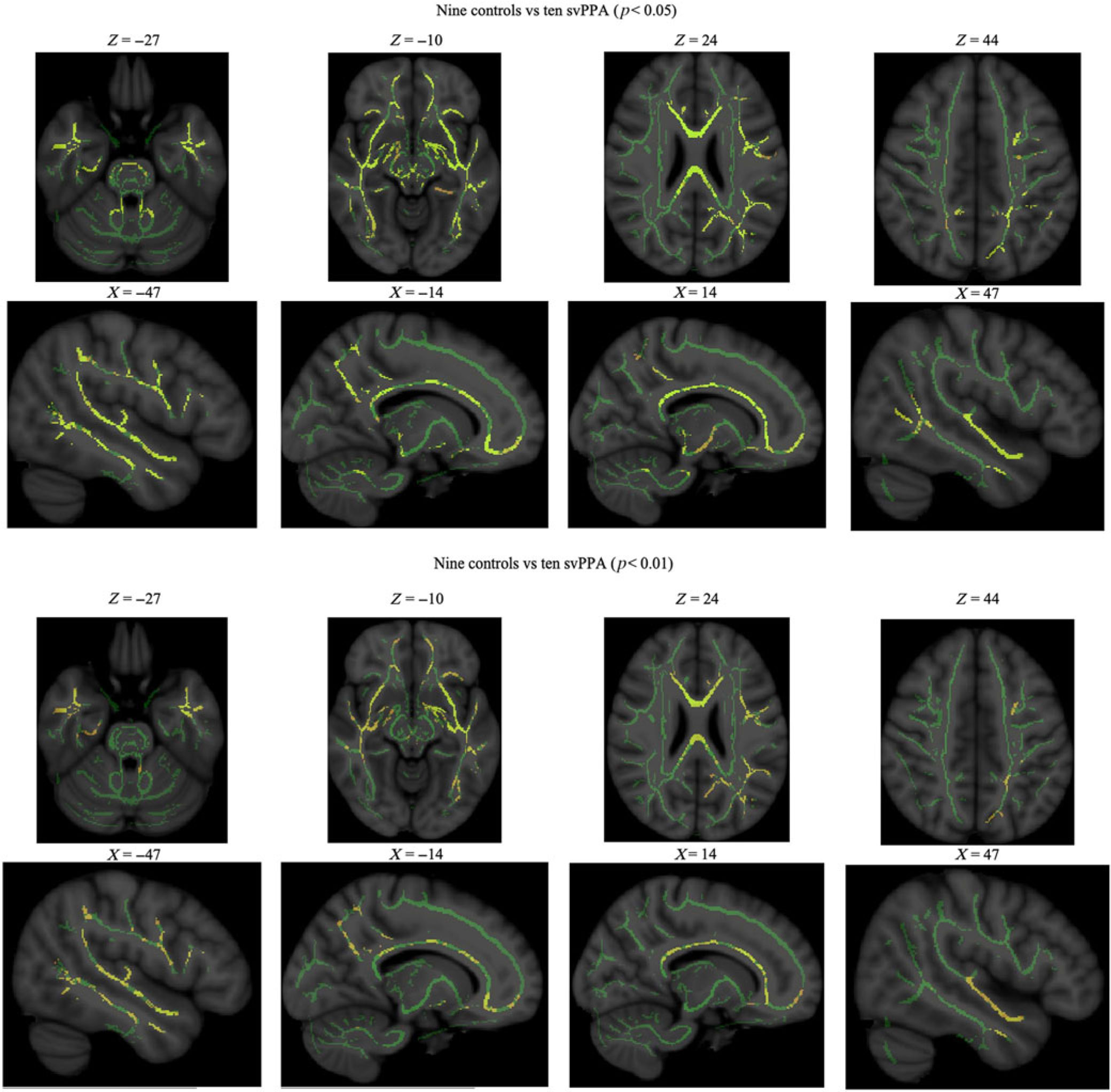
Figure 1: WM maps: statistically significant differences in fractional anisotropy between svPPA patients and controls. svPPA, semantic variant of primary progressive aphasia.
Table 2: WM study: fractional anisotropy data for svPPA patients and controls
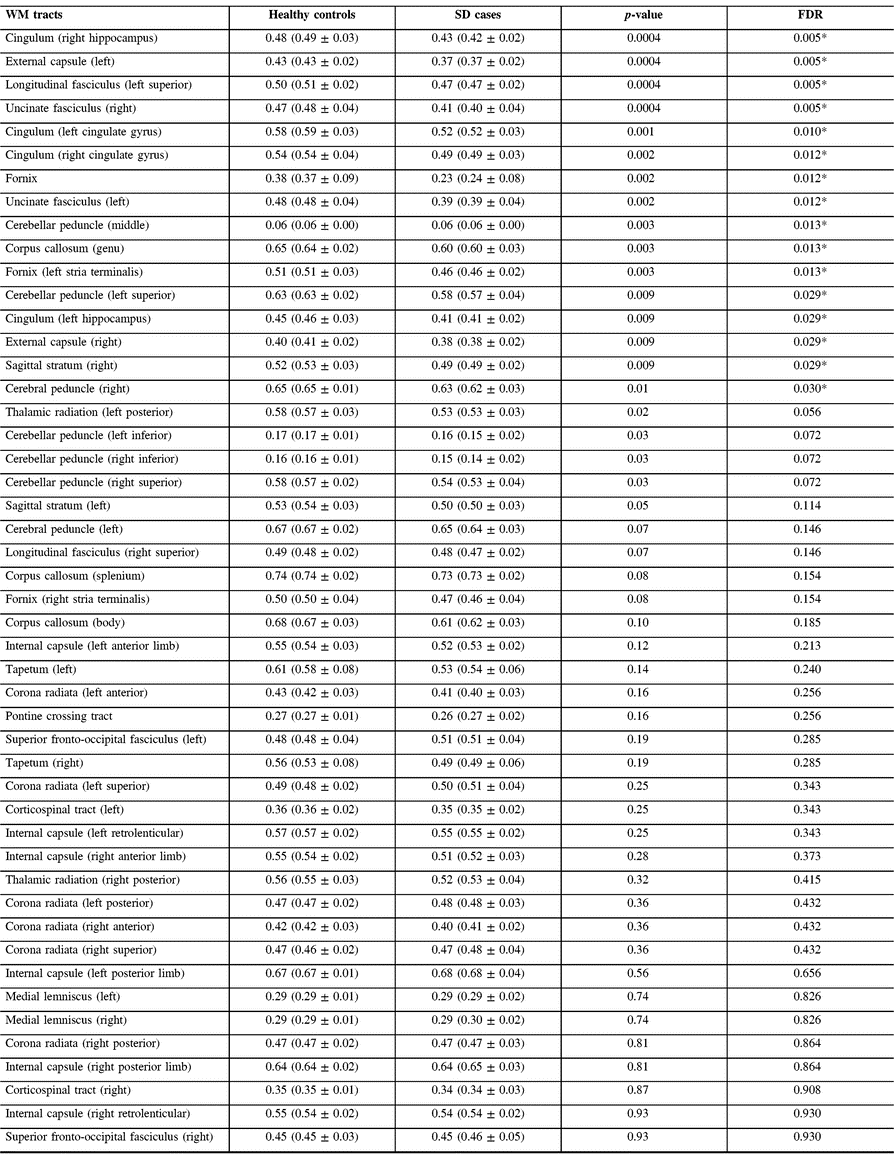
FDR = false discovery rate; svPPA = semantic variant of primary progressive aphasia; WM = white matter.
* p < 0.05 (false discovery rate corrected).
Results are presented as median (mean ± standard deviation).
GM Analysis
Volumetric data showed that both groups had indistinguishable total intracranial capacity and total brain volumes. However, our group of svPPA patients had smaller subcortical and left hemispheric cortical GM volumes. When looking at specific ROIs, volumes were consistently lower in svPPA patients than in healthy controls, reaching significance in 22 of the 33 studied regions, after FDR correction. The most significant differences were noted in the left amygdala, entorhinal, fusiform, inferior temporal, middle temporal and superior temporal gyri, as well as in the temporal poles and right fusiform GM (see Table 3 and Figure 2).
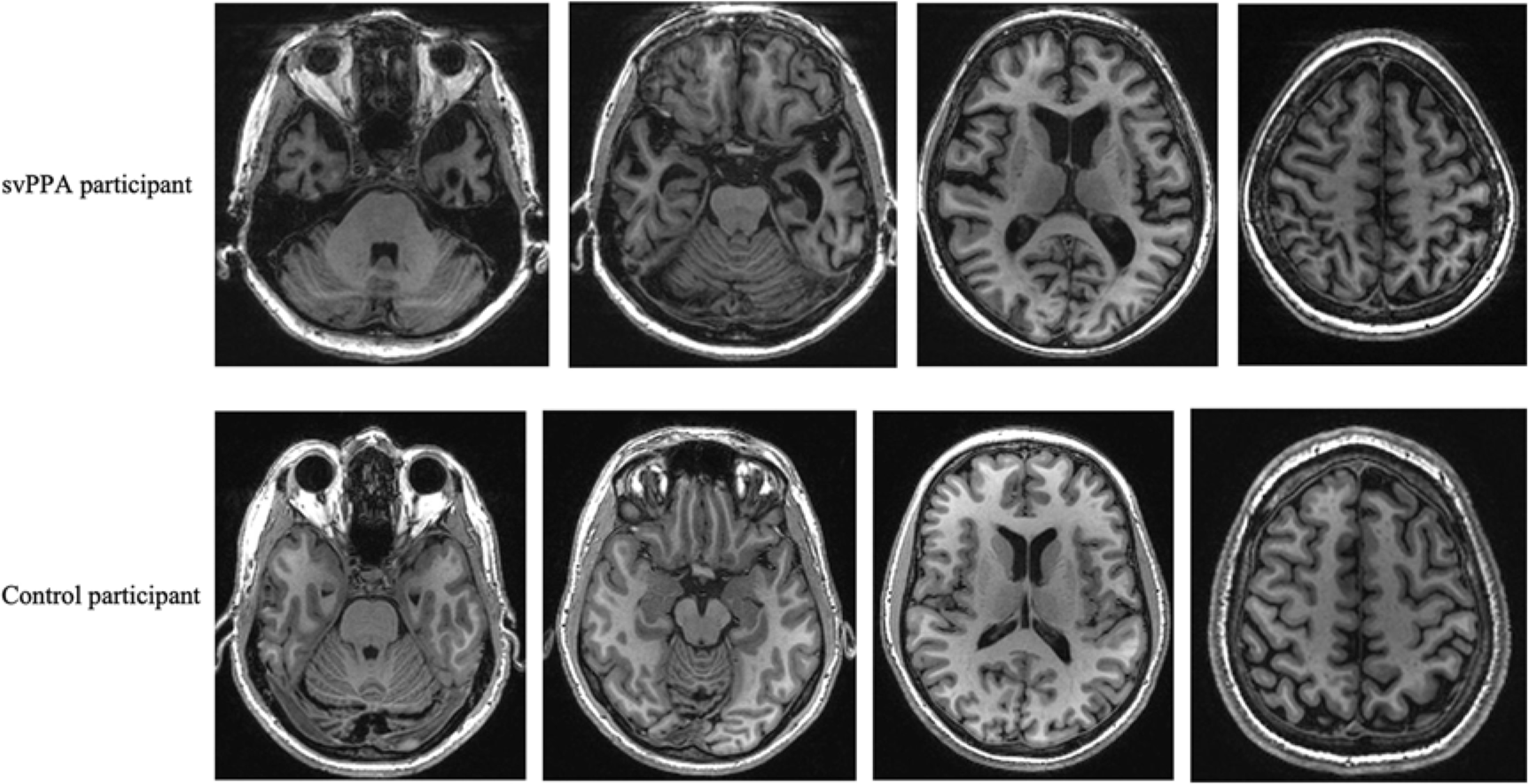
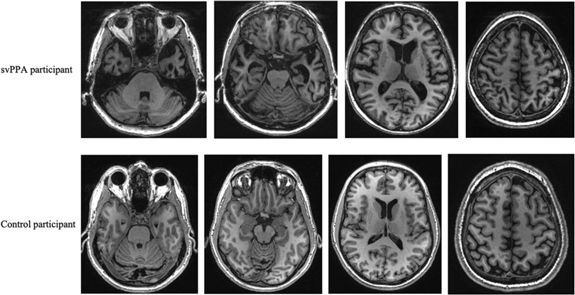
Figure 2: GM images: examples of atrophy in a svPPA patient compared with a cognitively healthy control. svPPA, semantic variant of primary progressive aphasia.
Table 3: GM study: volumetric data for svPPA cases and controls
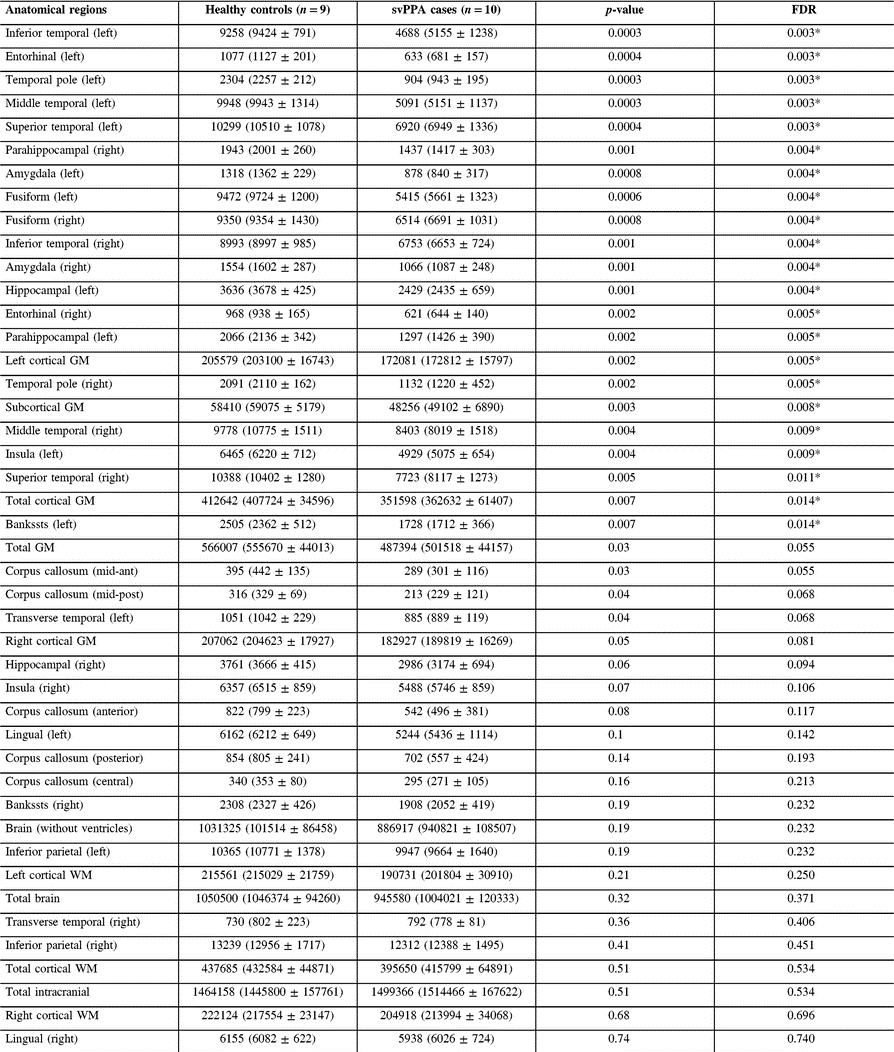
GM = grey matter; svPPA = semantic variant of primary progressive aphasia; Bankssts = banks of the superior temporal sulcus; WM = white matter.
* p < 0.05 (false discovery rate corrected).
Results are presented as median (mean ± standard deviation). Units are mm3.
Discussion
The semantic variant of primary progressive aphasia is a neurodegenerative disorder with language impairment as its hallmark. Previous literature reported cortical GM atrophy predominantly in the anterior temporal lobes, bilaterally but markedly in the left hemisphere. As for WM damage, literature is scant and based on a small number of studies and patients. Only a few reports have described abnormal diffusion, in one or more of the left postero-inferior longitudinal, uncinate, posterior cingulate fasciculi. Of these studies, only one used a more sensible tract-based approach instead of the ROI approach. We designed this study principally to address the knowledge gap we observed concerning WM damage in svPPA, in addition to validating the characterisation of GM atrophy. Our ultimate goal is to use such information to improve diagnosis, as we know that an early diagnosis might lead to earlier care and, in turn, decreased social difficulty.Reference Routhier, Gravel-Laflamme and Macoir10
Our first aim was to characterise cerebral WM lesions in our cohort of svPPA patients, compared with cognitively healthy, age-matched control subjects. Using a tract-based whole-brain approach, we found FA differences between our two groups, including statistically lower FA in the right hippocampus and uncinate fasciculus tract regions, as well as the left external capsule and superior longitudinal fasciculus tracts. Our second aim was to characterise cerebral GM lesions in these same patients. Using volumetric tools, we found significant atrophy differences between patients and controls. Notably, larger GM atrophy in svPPA patients was found in the left amygdala, in the left entorhinal, fusiform, inferior temporal, middle temporal and superior temporal gyri, left temporal pole and right fusiform gyrus.
Findings
Our WM results showed a significant diminution of FA in different tracts, which is known to demonstrate microstructural damages in these fasciculi. In accordance with some of the few reports available, we found impairment of the longitudinal fascicule and some of the uncinate fascicule;Reference Galantucci, Tartaglia and Wilson24, Reference Agosta, Scola and Canu26, Reference Mahoney, Malone and Ridgway27, Reference Agosta, Galantucci and Canu53 however, we also found that the damage was more extensive and less clearly lateralised than previously thought, including more extensive than GM atrophy. The latter may constitute an interesting line of inquiry as to the longitudinal progression of the disease.
Our GM results showed a lateralisation of atrophy related to svPPA, as it was more severe in the left hemisphere. We also found significant atrophy in the temporal lobes. These results are in line with those previously described in literature.Reference Wilson, Joubert and Ferré40 This is also in accordance with fundamental neuroimaging studies on the cerebral regions associated with language,Reference Wilson, Joubert and Ferré40 which have shown that the left anterior temporal lobe is part of the semantic brain network, whereas other functions like syntax would be processed more in the posterior temporal or frontal lobes.
Hypotheses
Two different hypotheses have been proposed to explain the pattern of WM damage.Reference Galantucci, Tartaglia and Wilson24, Reference Aghakhanyan, Aghakhanyan and Martinuzzi54–Reference Powers, McMillan and Brun59 The first one posits an extrinsic cause. Following GM atrophy by neuronal loss, a process of Wallerian degeneration or axonal degeneration begins, hence causing WM loss. Considering the severity of GM damage that we also observed in our cohort, this hypothesis would explain why not only tracts close to the left temporal lobe, but also other connecting tracts that are located more distantly, are impaired. The second hypothesis is an intrinsic direct axonal pathology.Reference Dagher and Nagano-Saito60 This explanation puts forward that intrinsic brain networks propagate WM disease, either by means of a process of neuroinflammation or by a cascade of misfolded proteins (prion-like),Reference Heneka, Carson and El Khoury61 an hypothesis put forward recently in a case of a novel prion protein variant in a patient with svPPA.Reference Kenny, Woollacott and Koriath62
Since our study is purely descriptive and cross-sectional, we cannot support firmly either hypothesis; however, the significantly large extent of WM atrophy would tend to support the intrinsic theory. Longitudinal studies are needed to understand the evolution of GM and WM damage in the brain, and hence confirm or infirm either hypothesis.
Strengths and Limitations
Compared with similar studies in the literature, and given the prevalence of svPPA in the population, the number of patients that we were able to recruit is a strength insofar as it allows us to compare directly with other studies of similar, or smaller, sizes, even though it still implied the use of non-parametric tests, with their inevitably lower statistical power. Furthermore, our inclusion and exclusion criteria prevented confounding factors from altering the internal validity of our results, while maximising participant recruitment. Finally, the whole-brain TBSS approach has many advantages such as correcting for misalignment, registration and smoothing, and removing the need to determine a priori ROI for the analysis, which would imply the use of a yet-incomplete knowledge of connectomics.
In contrast, there were some limitations arising from these same measurement techniques. The first comes from the use of DTI, as it is known that the diffusion tensor model has limited sensitivity to crossing fibres. Future studies could use high-angular-resolution diffusion imagingReference Descoteaux, Angelino and Fitzgibbons63 to alleviate this problem. The second limitation relating to the technique comes from the choice of FA as our sole metric to evaluate WM microstructural changes. The use of apparent diffusion coefficient, or axial, radial and mean diffusivity, could have improved the sensitivity of our study. It is indeed known that FA is a scalar value, and that different combinations of diffusion tensor eigenvalues that have changed concurrently could generate identical FA values.Reference Aghakhanyan, Aghakhanyan and Martinuzzi54 However, FA is a known, oft-reported metric that captures the essential changes related to WM integrity; employing other techniques could only uncover further areas or a deeper level of WM damages. Equally, TBSS will also be subject to reduced sensibility whenever fibres cross, for similar reasons as mentioned previously. Finally, the atlas used to extract numerical values remains composed of rather large structures that may end up masking small, sub-regional effects. The Human Connectome ProjectReference Van Essen, Ugurbil and Auerbach64 aims to improve our knowledge of WM tracts and is currently addressing this issue.
Our results in a French-speaking population are comparable with previous studies conducted on native speakers of other languages, mostly English. This means that, in terms of external validity, language does not seem to be a concern. This could lead to multicentric studies, without language barriers, involving a higher number of subjects in future studies, especially those on a longitudinal basis and those correlating clinical, anatomical and pathological data. As well, to better understand the notions of cerebral lateralisation and brain plasticity, it would also be of interest to include, as much as available, left-handed patients to assess if findings differ for this group.
Conclusion
In summary, our study confirms known GM atrophy in svPPA patients, as well as validates WM damage using a more sensitive and unbiased approach than previously reported. MRI and DTI appear to have the potential to occupy an important place in the initial investigation of dementia and could eventually be very useful in the differential diagnosis of atypical dementia, to characterise, as precisely as possible, the cerebral damage in one patient and to link it to a specific disease through its imaging signature.
Acknowledgements
All authors thank participants in the study for their dedication. MAW gratefully acknowledges financial support from the Réseau Québécois de Recherche sur le Vieillissement et Fonds de recherche du Québec – Société et culture # 168556). SD is a research scholar at Fonds de recherche du Québec – Santé.
Disclosures
The authors have no conflicts of interest to declare.
Authors’ Contributions
LOB: Study conception and design, analysis and interpretation of data, manuscript drafting and approval for publication.
MAW: Contribution to conception and design of study, interpretation of data, critical revision of manuscript for important intellectual content, and approval for publication.
RL: Patient recruitment, interpretation of data, critical revision of manuscript for important intellectual content, and approval for publication.
SD: Contribution to conception and design of study, interpretation of data, critical revision of manuscript for important intellectual content, and approval for publication.
All authors agree to be accountable for all aspects of the work.