Introduction
Childhood adversity (CA) refers to a range of negative experiences throughout childhood and adolescence such as parental psychopathology, peer victimization, and various forms of parental maltreatment (e.g., neglect or overt maltreatment). CA experiences are one of the strongest predictors of mental health problems (Green et al., Reference Green, McLaughlin, Berglund, Gruber, Sampson, Zaslavsky and Kessler2010), possibly through their impact on the developing brain. According to the social transactional model of psychiatric vulnerability (McCrory et al., Reference McCrory, Foulkes and Viding2022), CA experiences shape fronto-limbic development and related socio-emotional functioning to aid survival in high-threat environments. For instance, in the context of an abusive home-environment, it may be adaptive for a child to rapidly detect when a parent is angry, to expect negative feedback, and to adjust their behavior and emotions accordingly. However, in nonthreatening social environments such socio-emotional functioning adaptations may inadvertently evoke social problems and generate social stress, and ultimately lead to mental health problems in later life (McCrory et al., Reference McCrory, Foulkes and Viding2022).
Fortunately, not all individuals who have experienced CA develop mental illness; rather, a substantial proportion of individuals exposed to CA function resiliently later in life. Resilience refers to the capacity of a system (e.g., a brain, a child, a family, a community) to successfully adapt to challenges that threaten the function, survival, or development of that system (Masten et al., Reference Masten, Lucke, Nelson and Stallworthy2021; Masten & Monn, Reference Masten and Monn2015). Resilience in the context of CA, when the stressor has already taken place, refers to an outcome of positive mental health functioning on a given trajectory or at a given point in time. Such resilient functioning should be assessed across mental health domains, given the nonspecific negative impact of CA (Masten & Monn, Reference Masten and Monn2015), and should be better than others with similar severity of CA experiences (van Harmelen et al., Reference van Harmelen, Kievit, Ioannidis, Neufeld, Jones, Bullmore, Dolan and Goodyer2017). Resilient functioning in individuals exposed to CA is aided by an array of separate yet interrelated protective social and cognitive influences such as positive parenting, and social support, high self-esteem, low rumination (Fritz et al., Reference Fritz, de Graaff, Caisley, van Harmelen and Wilkinson2018; Kalisch et al., Reference Kalisch, Baker, Basten, Boks, Bonanno, Brummelman, Chmitorz, Fernàndez, Fiebach, Galatzer-Levy, Geuze, Groppa, Helmreich, Hendler, Hermans, Jovanovic, Kubiak, Lieb, Lutz and Kleim2017, Reference Kalisch, Cramer, Binder, Fritz, Leertouwer, Lunansky, Meyer, Timmer, Veer and van Harmelen2019; van Harmelen et al., Reference van Harmelen, Kievit, Ioannidis, Neufeld, Jones, Bullmore, Dolan and Goodyer2017, Reference van Harmelen, Blakemore, Goodyer and Kievit2021). Furthermore, it is thought that these socio-cognitive protective factors interact with brain structure and functioning (Ioannidis et al., Reference Ioannidis, Askelund, Kievit and van Harmelen2020). Recent reviews of the literature suggest that resilient functioning may be facilitated by larger hippocampal structure and increased functional connectivity between limbic regions and the central executive network (CEN) (Moreno-López et al., Reference Moreno-López, Ioannidis, Askelund, Smith, Schueler and van Harmelen2020). Although these studies provide important insights about the neurobiology that may aid resilient functioning, recent advances in neuroscience indicate that cognitive and emotional processes are not merely facilitated by specific regions but emerge through the interaction of brain networks (Krendl & Betzel, Reference Krendl and Betzel2022).
Brain networks can be constructed from structural or functional neuroimaging data (Krendl & Betzel, Reference Krendl and Betzel2022). Structural covariance networks are thought to overlap with functional networks (Zielinski et al., Reference Zielinski, Gennatas, Zhou and Seeley2010), and can be examined using graph theoretical approaches, such as structural covariance (Bullmore & Bassett, Reference Bullmore and Bassett2011; Kaiser, Reference Kaiser2011; Rubinov & Sporns, Reference Rubinov and Sporns2010). Structural covariance reflects the interindividual (Alexander-Bloch et al., Reference Alexander-Bloch, Raznahan, Bullmore and Giedd2013; Vijayakumar et al., Reference Vijayakumar, Ball, Seal, Mundy, Whittle and Silk2021) or intra-individual (Seidlitz et al., Reference Seidlitz, Váša, Shinn, Romero-Garcia, Whitaker, Vértes, Wagstyl, Kirkpatrick Reardon, Clasen, Liu, Messinger, Leopold, Fonagy, Dolan, Jones, Goodyer, Raznahan and Bullmore2018; Yun et al., Reference Yun, Kim, Lee, Chon and Kwon2016) covariation in brain morphology (for example cortical thickness) between different regions (nodes). Importantly, interindividual structural covariance may reflect coordinated brain development (Alexander-Bloch et al., Reference Alexander-Bloch, Raznahan, Bullmore and Giedd2013; Khundrakpam et al., Reference Khundrakpam, Reid, Brauer, Carbonell, Lewis, Ameis, Karama, Lee, Chen, Das and Evans2013; Vijayakumar et al., Reference Vijayakumar, Ball, Seal, Mundy, Whittle and Silk2021). During puberty and adolescence, the cerebral cortex becomes thinner (Wierenga et al., Reference Wierenga, Langen, Oranje and Durston2014) and white matter tracts become more densely myelinated (Miller et al., Reference Miller, Duka, Stimpson, Schapiro, Baze, McArthur, Fobbs, Sousa, Sěstan, Wildman, Lipovich, Kuzawa, Hof and Sherwood2012) suggesting a progressive refinement of neural connections through ongoing neural regressive events such as pruning (Kaiser, Reference Kaiser2011; Alexander-Bloch et al., Reference Alexander-Bloch, Raznahan, Bullmore and Giedd2013; Miller et al., Reference Miller, Duka, Stimpson, Schapiro, Baze, McArthur, Fobbs, Sousa, Sěstan, Wildman, Lipovich, Kuzawa, Hof and Sherwood2012; Zielinski et al., Reference Zielinski, Gennatas, Zhou and Seeley2010). The transition from childhood to adolescence is characterized by global increases in structural covariance of cortical thickness followed by reductions into mid-adolescence (Vijayakumar et al., Reference Vijayakumar, Mills, Alexander-Bloch, Tamnes and Whittle2018). Structural covariance continues to decrease through late adolescence before plateauing in the early twenties, which corresponds to the prolonged maturation of association cortices (Váša et al., Reference Váša, Seidlitz, Romero-Garcia, Whitaker, Rosenthal, Vértes, Shinn, Alexander-Bloch, Fonagy, Dolan, Jones, Goodyer, Sporns and Bullmore2018). The associated developmental changes in structural covariance during later adolescence, such as cortical thinning, have been related to reductions in nodal degree, the number of connections that brain regions have in a network (Váša et al., Reference Váša, Seidlitz, Romero-Garcia, Whitaker, Rosenthal, Vértes, Shinn, Alexander-Bloch, Fonagy, Dolan, Jones, Goodyer, Sporns and Bullmore2018). Such development is thought to be shaped by genetic as well as environmental influences (Whitaker et al., Reference Whitaker, Vértes, Romero-Garcia, Váša, Moutoussis, Prabhu, Weiskopf, Callaghan, Wagstyl, Rittman, Tait, Ooi, Suckling, Inkster, Fonagy, Dolan, Jones, Goodyer, Goodyer and Villis2016).
Reviews on the neurobiology of resilience show an overall lack of consistent findings which could be the result of different conceptualizations of resilience (Eaton et al., Reference Eaton, Cornwell, Hamilton-Giachritsis and Fairchild2022; Zhang et al., Reference Zhang, Rakesh, Cropley and Whittle2023; Leal & Silvers, Reference Leal and Silvers2021). In general, reviews point to neural circuits involved in emotion regulation and reward (Eaton et al., Reference Eaton, Cornwell, Hamilton-Giachritsis and Fairchild2022; Leal & Silvers, Reference Leal and Silvers2021), fronto-subcortical networks (Zhang et al., Reference Zhang, Rakesh, Cropley and Whittle2023) and the emotional brain (Moreno-López et al., Reference Moreno-López, Ioannidis, Askelund, Smith, Schueler and van Harmelen2020). To date, only a few studies have investigated the relationship between resilience in individuals exposed to CA and structural brain networks (reviewed in Moreno-López et al., Reference Moreno-López, Ioannidis, Askelund, Smith, Schueler and van Harmelen2020). Ohashi et al. (Reference Ohashi, Anderson, Bolger, Khan, McGreenery and Teicher2019) found reduced nodal efficiency in resilient individuals in the amygdala and 8 other nodes compared to susceptible individuals exposed to CA using diffusion tensor imaging and tractography. Comparing groups of non-maltreated youth, maltreated youth with PTSD and maltreated youth without PTSD, Sun et al. (Reference Sun, Haswell, Morey and De Bellis2019) found larger centrality (importance of a region within a network) in the right frontal pole in maltreated youth without PTSD symptomatology compared to non-maltreated youth and maltreated youth with PTSD based on a structural covariance derived from cortical thickness estimates. The frontal pole plays a role in adapting and updating reward processing models in response to the environment (Kovach et al., Reference Kovach, Daw, Rudrauf, Tranel, O'Doherty and Adolphs2012). In a study with a similar design, maltreated youth without PTSD (versus with PTSD) showed larger centrality in right orbitofrontal cortex (Sun et al., Reference Sun, Peverill, Swanson, McLaughlin and Morey2018), a region critical for evaluation, affect regulation and reward-based decision-making (Fettes et al., Reference Fettes, Schulze and Downar2017). Thus, resilient functioning in individuals exposed to CA is likely related to altered structural covariance patterns. However, these studies estimated resilience at the group-level, and were not able to relate the findings to individual level of resilient functioning, limiting the generalizability of these findings.
Hence, little is known about the structural network topology related to the level of resilient functioning in individuals exposed to CA, particularly in young people when the brain is in development. In doing so, appropriate quantification of resilient functioning after CA must keep in mind the following aspects. Firstly, given the negative impact of CA on a range of mental health and well-being outcomes, it is important that resilient functioning incorporates functioning across these psychological and social ("psychosocial") domains of functioning (Masten & Monn, Reference Masten and Monn2015). Such resilient functioning across domains should further take into account what someone has experienced, as individuals with similar psychosocial functioning may differ in their degree of resilient functioning when one has experienced more severe CA than the other. Finally, as CA is a highly clustered experience, where different types of adversity often co-occur, it is important to take all CA experiences into account when examining CA. To do so, we build on previous work (Ioannidis et al., Reference Ioannidis, Askelund, Kievit and van Harmelen2020; van Harmelen et al., Reference van Harmelen, Kievit, Ioannidis, Neufeld, Jones, Bullmore, Dolan and Goodyer2017, Reference van Harmelen, Blakemore, Goodyer and Kievit2021) and use data reduction techniques (principal component analyses) to derive a single estimate for psychosocial functioning that summarizes low to high functioning across multiple measurements, and use the same approach to calculate a single estimate that summarizes the severity of all experiences of childhood family adversity (CFA) in a community cohort of healthy young people with low to moderate CFA (N = 2406, aged 14–24). Next, we regressed the estimate for CA onto the estimate for psychosocial functioning. In doing so, individual-level resilient functioning can then be inferred from the residuals of the relation between CA and psychosocial functioning - the extent to which an individual is functioning better than expected given their CA experiences (implying resilient functioning, green lines) or worse than expected (implying vulnerable functioning, red lines) (Figure 1b, see Ioannidis et al., Reference Ioannidis, Askelund, Kievit and van Harmelen2020; van Harmelen et al., Reference van Harmelen, Kievit, Ioannidis, Neufeld, Jones, Bullmore, Dolan and Goodyer2017, Reference van Harmelen, Blakemore, Goodyer and Kievit2021). The aim of this study is to examine whether such resilient functioning in young people exposed to CFA is associated with altered structural network topology. To do so, we use a sliding window method (See Figure 1c); a novel approach so far only used to estimate the structural network topology of neurodevelopmental trajectories (Alexander-Bloch et al., Reference Alexander-Bloch, Raznahan, Bullmore and Giedd2013; Ohashi et al., Reference Ohashi, Anderson, Bolger, Khan, McGreenery and Teicher2019). Here, we use the sliding window method to be able to use resilient functioning as a continuous measure and test for robustness of its association with nodal degree by repeating the structural covariance analyses across different subsamples. By altering the window width and step size with each iteration, our findings get independent of the parameters used for the sliding window method. Given the importance of socio-emotional functioning in mental health vulnerability in adolescents and adults exposed to CA (McCrory et al., Reference McCrory, Foulkes and Viding2022; Moreno-López et al., Reference Moreno-López, Ioannidis, Askelund, Smith, Schueler and van Harmelen2020), we hypothesized that higher level of resilient functioning would be associated with changes in nodal degree of cortical brain regions that help guide socio-emotional functioning. Building on previous work in this sample (Váša et al., Reference Váša, Seidlitz, Romero-Garcia, Whitaker, Rosenthal, Vértes, Shinn, Alexander-Bloch, Fonagy, Dolan, Jones, Goodyer, Sporns and Bullmore2018; Whitaker et al., Reference Whitaker, Vértes, Romero-Garcia, Váša, Moutoussis, Prabhu, Weiskopf, Callaghan, Wagstyl, Rittman, Tait, Ooi, Suckling, Inkster, Fonagy, Dolan, Jones, Goodyer, Goodyer and Villis2016), we chose to investigate nodal degree in structural covariance networks derived from cortical thickness estimates since this measure was shown to decrease with age in adolescence, and this decrease was associated with corresponding myelination changes in the association cortices (Váša et al., Reference Váša, Seidlitz, Romero-Garcia, Whitaker, Rosenthal, Vértes, Shinn, Alexander-Bloch, Fonagy, Dolan, Jones, Goodyer, Sporns and Bullmore2018). Furthermore, we focused on CFA rather than the broader CA as our available data contains questionnaires that focus on the family environment. Data on other experiences of CA, such as bullying, racism, or poverty, were not included here.

Figure 1. Study design A) A covariance matrix of the cortical thickness (CT) measures for 308 parcellations in 275 participants is created. Next, the data matrix is substituted by the residuals of the linear regression to remove variation related to age, gender, and intra-cranial volume. B) Next, resilient functioning scores are created based on the NSPN sample (N = 2406). The figure shows the extent to which an individual functioned better than expected ("high, or resilient;" green lines), or worse than expected ("low or risk" red lines), than others with similar childhood family adversity experiences. Note that higher residual scores reflect more resilient functioning, and that both X and Y axes represent factor scores with Mean = 0 and SD = 1. C) Next, a sliding window method was applied with varying window sizes (red box), to assess how resilient functioning was related to changes in the nodal degree of the network overlapping structural networks of 275 participants. CT values of each region were cross correlated with windows containing the same numbers, we used bootstrapping to threshold the network. D) Then, we evaluated linear regional changes in node degree as a function of the median resilient functioning. E) We varied these parameters to explore consistency in the results using a “Convergence Index,” considering all nine combinations of window widths (40, 60, 80) and step sizes (5, 10, 20), plus one further combination of ww = 60 and ss = 30. Convergence indices were calculated for each of the brain parcellations, where the index represents the number of times the region is associated with resilient functioning for each of the above combinations. Thus, a convergence index of 10 indicates that the region was associated with resilient functioning every run and a convergence index of 0 indicates that it was never associated.
Methods
Study design and participants
Participants were part of the Neuroscience in Psychiatry Network (NSPN) study: a multi-center accelerated longitudinal community cohort study focusing on normative adolescent to young adult development between the ages 14–24. The NSPN cohort (N = 2406) was recruited from schools, colleges, National Health Service primary care services, and direct advertisement in north London and Cambridgeshire. Maintaining the same gender and ethnicity balance as in the main sample, 301 participants were invited for an MRI scan (Whitaker et al., Reference Whitaker, Vértes, Romero-Garcia, Váša, Moutoussis, Prabhu, Weiskopf, Callaghan, Wagstyl, Rittman, Tait, Ooi, Suckling, Inkster, Fonagy, Dolan, Jones, Goodyer, Goodyer and Villis2016). For this manuscript, we excluded those individuals with a lifetime history of brain damage, epilepsy, genetic syndromes, and premature birth (N = 26), leaving a total sample of 275 individuals for the analyses of brain imaging data below.
The inclusion criteria for the MRI subset were that the participants should be aged between 14 and 24 years; able to understand written and spoken English; have normal or corrected-to-normal vision; and able to give informed consent for participation in the study. The exclusion criteria were current treatment for psychiatric disorders, drug dependance, alcohol dependance, current or previous neurological disorders, brain trauma including epilepsy, head injury causing loss of consciousness, learning disability requiring specialist educational support and/or medical treatment, and standard MRI contraindications. Individuals with previous or lifelong psychiatric disorders were not excluded, except if they were in current treatment for these disorders. The study was approved by the Cambridgeshire and Peterborough Foundation Trust and the University of Cambridge research ethics committees (REF 12/EE/0250). All participants (and their caregivers) were briefed about the study aims and protocols and signed an informed consent form.
Acquired data
To assess resilient functioning, we relied on data from questionnaires on psychological functioning, CFA, socio-demographic status, family and educational or occupational environments, and subclinical psychopathology in the NSPN sample (N = 2406). Assessments for the MRI sub study (N = 275) further included a day of clinical, cognitive, and MRI assessments at the University of Cambridge or University College London sites.
Measures of psychosocial functioning
Psychosocial functioning was based on all measures included in the NSPN home questionnaire pack that assessed any aspect of psychological and social functioning. This included measures of psychiatric symptomatology, personality traits, and mental well-being. Below we provide an overview of the specific measures used, please refer to van Harmelen et al. (Reference van Harmelen, Kievit, Ioannidis, Neufeld, Jones, Bullmore, Dolan and Goodyer2017) and Supplementary material for a description of the measures. Psychosocial functioning was assessed with sum scores from the Mood and Feelings Questionnaire (MFQ; [Angold et al., Reference Angold, Costello, Van Kämmen and Stouthamer-Loeber1996]), Revised Children’s Manifest Anxiety Scale (RCMAS; [Reynolds & Richmond, Reference Reynolds and Richmond1997]), Short Leyton Obsessional Inventory (S-LOI; [Bamber et al., Reference Bamber, Tamplin, Park, Kyte and Goodyer2002]), Child Behavior Checklist (CBCL; [Achenbach, Reference Achenbach1991]) and Kessler Psychological Distress Scale (K10; [Kessler et al., Reference Kessler, Andrews, Colpe, Hiripi, Mroczek, Normand, Walters and Zaslavsky2002]), the Adolescent Psychopathy Screening Device (APSD; [Frick et al., Reference Frick, Bodin and Barry2000]), Child and Adolescent Dispositions Scale (CADS; [Lahey et al., Reference Lahey, Applegate, Chronis, Jones, Williams, Loney and Waldman2008]), the Inventory of Callous-Unemotional Traits (ICU; [Roose et al., Reference Roose, Bijttebier, Decoene, Claes and Frick2010]), Schizotypal Personality Questionnaire (SPQ; [Raine, Reference Raine1991]), and the Barratt Impulsiveness Scale (BIS-11; [Stanford et al., Reference Stanford, Mathias, Dougherty, Lake, Anderson and Patton2009]), and the Warwick-Edinburgh Mental Well-being Scale (WEMWBS; [Tennant et al., Reference Tennant, Hiller, Fishwick, Platt, Joseph, Weich, Parkinson, Secker and Stewart-Brown2007]).
Measures of childhood family adversity
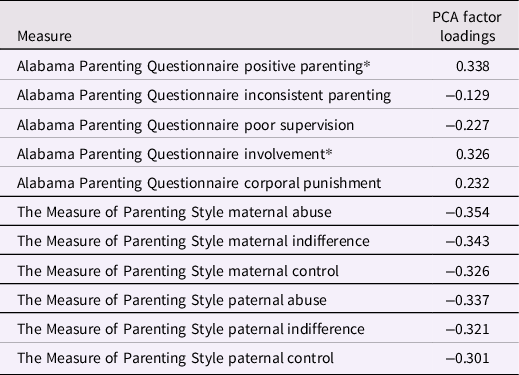
Childhood Family Adversity (CFA) scores included all measures in the NSPN home questionnaire pack that assessed any aspect of the home environment whilst growing up. As such, CFA within the family environment was assessed with the Alabama Parenting Questionnaire (APQ; [Elgar et al., Reference Elgar, Waschbusch, Dadds and Sigvaldason2007]) and the Measure of Parental Style (MOPS; [Parker et al., Reference Parker, Roussos, Hadzi-Pavlovic, Mitchell, Wilhelm and Austin1997]). The types of experiences assessed with the APQ and MOPS include parental abuse and neglect, and more general parenting behaviors (i.e., positive parenting, inconsistence, indifference, and control). Please refer to van Harmelen et al. (Reference van Harmelen, Kievit, Ioannidis, Neufeld, Jones, Bullmore, Dolan and Goodyer2017) and Supplementary material for a description of the measures.
MRI acquisition
Participants underwent structural MRI (3T) using the multi-parameter mapping (MPM) sequence (Weiskopf et al., Reference Weiskopf, Suckling, Williams, Correia, Inkster, Tait, Ooi, Bullmore and Lutti2013) in Cambridge (two sites) or London. All sites used identical scanners (Siemens Magnetom Tim Trio), sequences, and protocols. The setup, acquisition, and post-processing have been previously described (Weiskopf et al., Reference Weiskopf, Suckling, Williams, Correia, Inkster, Tait, Ooi, Bullmore and Lutti2013). Briefly, the MPM protocol includes three multi-echo 3D FLASH (fast low-angle shot) scans, one radiofrequency (RF) transmit field map, and one static magnetic (B0) field map scan. Multiple gradient echoes were acquired with alternating readout polarity at six equidistant echo times between 2.2 and 14.7 ms for both acquisitions. Other acquisition parameters were: 1 mm isotropic resolution, 176 sagittal partitions, field of view = 256 × 240 mm, matrix = 256 × 240 × 176, parallel imaging using GRAPPA factor 2 in phase-encoding direction (AP), 6/8 partial Fourier in partition direction, nonselective RF excitation, readout bandwidth BW = 425 Hz/pixel, RF spoiling phase increment = 50 Å. The total acquisition time was ∼25 min. Participants were instructed to lie still and rest during the scan.
MRI processing
MR images were processed using the Freesurfer pipeline (v5.3.0), including skull-stripping, and segmentation of cortical gray and white matter (Fischl et al., Reference Fischl, Salat, Busa, Albert, Dieterich, Haselgrove, van der Kouwe, Killiany, Kennedy, Klaveness, Montillo, Makris, Rosen and Dale2002). After quality control, three participants were excluded from further analysis because of movement artifacts, which prevented accurate surface reconstructions and reconstruction of the cortical surface and gray-white matter boundary (for more detail see Whitaker et al., [Reference Whitaker, Vértes, Romero-Garcia, Váša, Moutoussis, Prabhu, Weiskopf, Callaghan, Wagstyl, Rittman, Tait, Ooi, Suckling, Inkster, Fonagy, Dolan, Jones, Goodyer, Goodyer and Villis2016]). Parcellation of cortical gray matter regions was based on the anatomical borders of 308 equally sized regions (159 in each hemisphere) of 500 mm2 that were constrained by the anatomical boundaries according to the Desikan-Killiany atlas (Desikan et al., Reference Desikan, Ségonne, Fischl, Quinn, Dickerson, Blacker, Buckner, Dale, Maguire, Hyman, Albert and Killiany2006). We opted to use this particular atlas because it comprises an optimal spatial scale for graph theory analysis (Romero-Garcia et al., Reference Romero-Garcia, Atienza, Clemmensen and Cantero2012). Average CT was extracted for each of the 308 regions in each participant.
Analyses
Missing data handling
All analyses were conducted using R version 3.4.1 "Single Candle" of the Lavaan package (Rosseel, Reference Rosseel2012). From the NSPN cohort (N = 2406), all behavioral measures were complete for 1907 participants. The subset with no missing data did not differ from the larger sample in terms of age (t(4091) = −0.027, p = 0.97), gender (χ 2 = 0.01, df = 1, p = 0.92), socioeconomic status (SES; index of multiple deprivation based on participant postcodes; t(4038) = −1.29, p = 0.19), or ethnicity (χ 2 = 3.65, df = 5, p = 0.60). Thus, missing data on the measures used in the below analyses were imputed using the Amelia package in R (Honaker et al., Reference Honaker, King and Blackwell2011). We calculated resilient functioning scores (as per the below description) within all 5 imputed datasets. These resilient functioning scores were highly correlated (r’s > 0.9, see supplemental Table S1 for specifics). Therefore, for this manuscript, we used the resilient functioning scores that were calculated using data from the first imputation sample.
Resilient functioning scores
Following the procedure detailed in van Harmelen et al. (Reference van Harmelen, Kievit, Ioannidis, Neufeld, Jones, Bullmore, Dolan and Goodyer2017), we estimated resilient functioning using the "residual method" on the imputed dataset for the NSPN cohort (N = 2406). Please refer to Ioannidis et al. (Reference Ioannidis, Askelund, Kievit and van Harmelen2020) for a detailed discussion of the benefits and drawbacks of this approach to quantify resilient functioning, and Cahill et al. (Reference Cahill, Hager and Chandola2022) for external validation of this approach which has shown good psychometric properties. Using this method we previously showed that adolescent friendships predict resilient functioning in two large independent samples; the N = 2406 NSPN sample (van Harmelen et al., Reference van Harmelen, Kievit, Ioannidis, Neufeld, Jones, Bullmore, Dolan and Goodyer2017), and in the N = 1238 Roots sample (van Harmelen et al., Reference van Harmelen, Blakemore, Goodyer and Kievit2021). We used principal component analysis (PCA) to compute individual psychosocial functioning scores using standard-normally transformed individual total scores across a range of measures (see Table 1; MFQ, RCMAS, S-LOI, K10, CBCL, APSD, CADS, ICU, SPQ, BIS-11, and WEMWBS). We also utilized PCA to calculate individual levels of CFA severity using standard-normally transformed sum scores for the MOPS and the APQ subscales (see Table 1) within the entire NSPN sample (N = 2409). Resulting CFA factor scores were regressed onto the psychosocial functioning factor scores, and the best fitting regression model (in this case, quadratic) was obtained. The residuals from this model reflect how much better or worse individuals are functioning when compared to others with similar CFA scores. As such, these residual scores can be interpreted as a proxy to indicate individual degree of "vulnerable to resilient" functioning (from here, for brevity we refer to this as "resilient functioning"), with higher scores reflecting better psychosocial functioning relative to the level of CFA. Next, individual resilient functioning scores were extracted for the MRI cohort (N = 275) and utilized in subsequent analyses.
Table 1. Factor loadings for the PCA’s for psychosocial functioning

*High score indicates positive psychosocial functioning.
Structural covariance
To estimate each structural network, we used Pearson’s correlation coefficients on the cortical thickness (CT) values estimated from Freesurfer based on structural MRI. Cortical thickness estimates were corrected for intracranial volume. Further, we performed a linear regression on regional CT values to remove effects of age and gender on cortical thickness. The residuals of this regression then replaced the raw values in the CT data matrix. These detrending steps were implemented to remove potentially confounding interindividual variation related to age and gender and intracranial volume. This method has been utilized by others (Melie-Garcia et al., Reference Melie-Garcia, Slater, Ruef, Sanabria-Diaz, Preisig, Kherif, Draganski and Lutti2018). Next, we used bootstrapping to threshold the network. Using this method, and for each window, an equal number of participants were resampled with replacement to construct 1000 bootstrapped structural networks. We then examined whether there were significant relations between each pair of regions across all bootstrapped networks. Consistent relationships between a pair of regions (at p < .001 adjusted for the False Discovery Rate (FDR) at the pair level (Váša et al., Reference Váša, Seidlitz, Romero-Garcia, Whitaker, Rosenthal, Vértes, Shinn, Alexander-Bloch, Fonagy, Dolan, Jones, Goodyer, Sporns and Bullmore2018)) were retained and the remaining relationships were discarded. For comparison, the main analyses were repeated without the detrending step for age and gender (Supplement).
Sliding window method
We applied a sliding window method to assess how resilient functioning was related to changes in the nodal degree of the network, defined as the number of edges connected to a node (Figure 1). CT values of each region were cross-correlated with windows containing the same numbers of participants and moved across resilient functioning scores by stepwise increases. At each step (within each window) we estimated a structural covariance network. For more information on the sliding window technique see Váša et al. (Reference Váša, Seidlitz, Romero-Garcia, Whitaker, Rosenthal, Vértes, Shinn, Alexander-Bloch, Fonagy, Dolan, Jones, Goodyer, Sporns and Bullmore2018). The selection of sliding window parameters, including window width (ww) and step size (ss) in units of number of participants involved several tradeoffs. The number of windows was defined using the following equation:
Where N part was the number of participants (n = 275), and ceil the ceiling function, which rounds non-integer fractions to the smallest integer larger than said fraction. The ss and ww define the number of windows and this in turn has an impact on the sensitivity of the analyses. Therefore, we varied these parameters to explore consistency in the results, considering all nine combinations of window widths (40, 60, 80) and step sizes (5, 10, 20), plus one further combination of ww = 60 and ss = 30, overlapping structural networks of 275 participants. We assessed the network topology changes by measuring the nodal degree. We then evaluated linear regional changes in nodal degree as a function of the median resilient functioning with Akaikes information criterion (AIC) and corrected for multiple comparisons (adjusted for the false discovery rate, p < .05). We ran permutation tests at the level of the windows without reconstructing the correlation matrices to see if the regional effects of change in structural correlation as a function of resilient functioning was valid. A convergence index was calculated for each of the brain region under consideration, where the index represents the number of times the region is associated with resilient functioning for each of the above combinations. Thus, the convergence index of a cortical region 10 indicates that the region is always associated with resilient functioning and the convergence value of 0 of another region (or node) indicates that it never is (see Figure 1). Next, we conducted a standardization to define the significant regions associated with resilient functioning (z > 1.645).
Availability of data and code
Data and code for the analyses will be available upon reasonable request at the University of Cambridge repository (https://www.repository.cam.ac.uk/). We uploaded the main results in the neuroimaging repository neurovault.org (Gorgolewski et al., Reference Gorgolewski, Varoquaux, Rivera, Schwarz, Ghosh, Maumet, Sochat, Nichols, Poldrack, Poline, Yarkoni and Margulies2015). The 1_6CI.nii.gz upload (https://identifiers.org/neurovault.image:785762) corresponds to the mean of the ß-estimates after thresholding of the main effect of resilient functioning on local nodal degree (Figure 2).

Figure 2. Brain parcellations with positive or negative correlations between nodal degree and resilient functioning. Cortical regions where resilient functioning was significantly convergently associated with nodal degree after thresholding based on the convergence index (z > 1.645). The colorbar represents the mean of the β-estimates with positive correlations in red and negative correlations in blue.
Results
Principal component scores
The first principal component of PCA for psychosocial functioning explained 44% variance across all psychological functioning measures (SD = 2.41, see Table 1 for all factor loadings). A higher score on the first component score was related to poorer psychosocial functioning, therefore, individual scores were subsequently inverted so that higher scores would indicate better psychosocial functioning.
The PCA for CFA resulted in a first component score that explained 37% variance (SD = 2.02, see Table 2 for specifics). Here, higher scores were related to lower CA and were subsequently inverted to indicate more CFA. As the score for CFA included a few positive parenting scales, we repeated the PCA without the positive parenting subscale. After removal of this subscale, the explained variance of our principal component was reduced by 0.3% (from 37.2% to 36.9%). Furthermore, principal component scores with and without this subscale correlated highly (r = .98, t = −331.5, df = 2404, p-value < 2.2e-16). Therefore, we decided to leave the positive parenting subscales in the PCA, in line with previous work (van Harmelen et al., Reference van Harmelen, Kievit, Ioannidis, Neufeld, Jones, Bullmore, Dolan and Goodyer2017).
Table 2. Factor loadings for the PCA’s for childhood family adversity
*High score indicates positive childhood family experiences.
Other components were observed in the data, however, the variance explained by these components was not enough to warrant additional analyses for any of these components in isolation. Scree plots showing the explained variance for each component in both PCA’s can be found in the Supplement (Figure S1). The supplement also includes descriptive statistics for all subscales included in the PCA and demographic variables (age, gender and ethnicity) are listed (Table S3).
Resilient functioning
To quantify the level of resilient functioning in our sample, we regressed the factor scores for CFA onto the factor score for psychosocial functioning. A linear model provided good fit (adjusted R-squared = 0.28, F(1,2404) = 957.2, p < 2.2e-16, Est = −06.36e-01, SE = 2.05e-02, t = −30.94, p < 2e-16, AIC = 10,257.64). A quadratic term improved model fit (Est = −0.04, SE = 0.005, t = 7.09, p = 1.73e-12, AIC = 10,204.58), SSM = 204.24, F(1) = 50.3, p < 1.73e-12). A further cubic model showed weak model fit (Est = −0.002, SE = 0.001, t = −1.98, p = .05), and only a minimal improvement (AIC = 10,202.68, SSM = 15.82 F(1) = 3.90, p = .05). Therefore, a quadratic model was selected (Figure 1b). Residual scores for this relationship were extracted as they reflect individual degrees of resilient functioning and were utilized in the subsequent analyses within the subsample from NSPN that underwent MRI (N = 275). These resilient functioning scores were normally distributed in the subsample with MRI data, and there were no significant relationships between estimated resilient functioning and age (p = 0.15), gender (p = 0.97), SES (p = 0.36), or scanning location (p = 0.9) (see Supplementary Figure S2).
Main results
Upon standardization (z > 1.65) of those regions that were convergently related to resilient functioning, we found that resilient functioning was associated with a decrease in the nodal degree (p < .05 FDR) of the posterior superior temporal sulcus (PSTS), dorsolateral prefrontal cortex (DLPFC), medial prefrontal cortex (MPFC), inferior and middle temporal gyrus (ITG and MTG), lateral occipital cortex (LOCC), pericalcarine, and premotor cortex (Figure 2). Resilient functioning was also related to an increase in the nodal degree (p < .05 FDR) of the anterior fusiform gyrus (FG) (Figure 2).
Sensitivity analysis
To investigate the impact of the statistical correction of regional CT values for age and gender, we next reestimated structural networks using raw CT values (uncorrected for age and gender, see supplemental material for details). When CT was uncorrected, our findings remained largely similar (Figure S3). Resilient functioning was positively associated with nodal degree in the DMPFC, PSTS, MPFC, left temporal pole, lateral occipital, and lingual gyrus and negatively associated with nodal degree in the FG. In addition, our findings now also included positive associations between resilient functioning and nodal degree in the left temporoparietal junction (superior parietal and supramarginal gyrus).
Discussion
The aim of this study was to investigate how brain structural network topology varies as a function of resilient functioning in a sample of adolescents and young adults with CFA. We showed that resilient psychosocial functioning is negatively associated with nodal degree of the dorsolateral prefrontal cortex (DLPFC), the medial prefrontal cortex (MPFC), the posterior superior temporal sulcus (PSTS), the inferior and middle temporal gyrus (ITG and MTG, resp.), the lateral occipital cortex (LOCC), the pericalcarine cortex, and the premotor cortex. These regions all play a role in a wide array of functions. Of particular interest is the role of these regions in social and emotional processing and regulation, given the importance of socio-emotional functioning in mental health vulnerability in adults exposed to CA (McCrory et al., Reference McCrory, Foulkes and Viding2022). The DLPFC is part of the CEN and involved in cognitive control and emotion regulation (Ochsner et al., Reference Ochsner, Bunge, Gross and Gabrieli2002, Reference Ochsner, Silvers and Buhle2012). Whereas the MPFC plays an important role in understanding social emotions and mentalizing (Blakemore, Reference Blakemore2008; Olson et al., Reference Olson, McCoy, Klobusicky and Ross2013; Van Overwalle, Reference Van Overwalle2009). Importantly, apart from its role in social functioning alterations in medial prefrontal cortex (mPFC)-subcortical circuitry after CFA are associated with a wide array of affective and cognitive functions (Tottenham, Reference Tottenham2020). Furthermore, the MPFC and temporal cortex are part of the default mode network (DMN, (Dixon et al., Reference Dixon, Thiruchselvam, Todd and Christoff2017)), which is thought to underpin introspective processes such as emotional processing, decision-making, memory, social cognition, and self-referential processes such as thinking about self-mental states (Northoff et al., Reference Northoff, Heinzel, de Greck, Bermpohl, Dobrowolny and Panksepp2006; Qin & Northoff, Reference Qin and Northoff2011). The DMN is also involved in thinking about other people’s beliefs, intentions, and motivations (Koster-Hale & Saxe, Reference Koster-Hale and Saxe2013; Spreng et al., Reference Spreng, Mar and Kim2009). The PSTS has been identified as an important hub in social cognitive processing at different levels, integrating advanced associative and lower-level sensory processing areas (Allison et al., Reference Allison, Puce and McCarthy2000). As such, our findings of reduced nodal degree of regions related to resilient functioning point to regions that help guide socio-emotional functioning.
During adolescence, the brain undergoes remarkable structural and functional reorganization, such as cortical thinning (Frangou et al., Reference Frangou, Modabbernia, Williams, Papachristou, Doucet, Agartz, Aghajani, Akudjedu, Albajes-Eizagirre, Alnæs, Alpert, Andersson, Andreasen, Andreassen, Asherson, Banaschewski, Bargallo, Baumeister, Baur-Streubel and Dima2022; Tamnes et al., Reference Tamnes, Herting, Goddings, Meuwese, Blakemore, Dahl, Güroğlu, Raznahan, Sowell, Crone and Mills2017; Wierenga et al., Reference Wierenga, Langen, Oranje and Durston2014), a decrease in cortical surface area and cortical volume (Tamnes et al., Reference Tamnes, Herting, Goddings, Meuwese, Blakemore, Dahl, Güroğlu, Raznahan, Sowell, Crone and Mills2017) and increases in white matter volume (Giedd et al., Reference Giedd, Blumenthal, Jeffries, Castellanos, Liu, Zijdenbos, Paus, Evans and Rapoport1999). On the microstructural level, such increases in white matter integrity and reorganization of structural brain networks are thought to contribute to more efficient structural brain networks (Koenis et al., Reference Koenis, Brouwer, van den Heuvel, Mandl, van Soelen, Kahn, Boomsma and Hulshoff Pol2015). Resting-state functional connectivity shows age-related increases within networks and decreases between networks (Teeuw et al., Reference Teeuw, Brouwer, Guimarães, Brandner, Koenis, Swagerman, Verwoert, Boomsma and Hulshoff Pol2019). In the current sample, decreases in nodal degree have been associated with the pruning of synaptic connections or attenuation of axonal projections in adolescence (Váša et al., Reference Váša, Seidlitz, Romero-Garcia, Whitaker, Rosenthal, Vértes, Shinn, Alexander-Bloch, Fonagy, Dolan, Jones, Goodyer, Sporns and Bullmore2018) and associated cortical thinning as well as the associated increases in myelination in these regions (Whitaker et al., Reference Whitaker, Vértes, Romero-Garcia, Váša, Moutoussis, Prabhu, Weiskopf, Callaghan, Wagstyl, Rittman, Tait, Ooi, Suckling, Inkster, Fonagy, Dolan, Jones, Goodyer, Goodyer and Villis2016). As such, decreases in nodal degree are thought to reflect a normative developmental shift to a more efficient brain network configuration (Khundrakpam et al., Reference Khundrakpam, Reid, Brauer, Carbonell, Lewis, Ameis, Karama, Lee, Chen, Das and Evans2013; Váša et al., Reference Váša, Seidlitz, Romero-Garcia, Whitaker, Rosenthal, Vértes, Shinn, Alexander-Bloch, Fonagy, Dolan, Jones, Goodyer, Sporns and Bullmore2018). Decreased nodal degree of brain regions in more resilient adolescents thus potentially resembles a mature structural network configuration in these individuals. Social support is known to influence adaptive maturational patterns; stronger mother-child interactions are associated with more mature prefrontal-limbic connectivity patterns in children (Gee et al., Reference Gee, Gabard-Durnam, Telzer, Humphreys, Goff, Shapiro, Flannery, Lumian, Fareri, Caldera and Tottenham2014), and friendship increases are associated with faster cortical thinning in the mPFC in adolescents (Becht et al., Reference Becht, Wierenga, Mills, Meuwese, van Duijvenvoorde, Blakemore, Güroğlu and Crone2021). Friendships support has been well-established as predictor of resilient functioning in adolescents and young adults exposed to CA (Fritz et al., Reference Fritz, de Graaff, Caisley, van Harmelen and Wilkinson2018; van Harmelen et al., Reference van Harmelen, Kievit, Ioannidis, Neufeld, Jones, Bullmore, Dolan and Goodyer2017; Reference van Harmelen, Blakemore, Goodyer and Kievit2021). Taking together the evidence of previous studies in the same and different samples, we suggest that our findings of negative associations between nodal degree and resilient functioning may reflect a more mature-like structural network topology in more resilient individuals. However, as we did not include longitudinal data, we can only speculate that developmental mechanisms explain the found associations. As such, it should be examined whether lower nodal degrees in individuals with more resilient functioning reflect more mature structural brain topology and or distinct developmental trajectories of the DLPFC, MPFC, PSTS, ITG, and MTG.
Resilient functioning was also associated with increased nodal degree in the anterior FG. One interpretation of our finding would be that maturation of the FG might be protracted (Haist & Anzures, Reference Haist and Anzures2017) or that this structure may be less mature in resilient individuals in this age group. The FG is specialized in the processing of faces and involved in facial emotion recognition (Adolphs, Reference Adolphs2002). Aberrant neural activation in response to emotional faces in individuals exposed to CA is consistently reported in literature (Bérubé et al., Reference Bérubé, Turgeon, Blais and Fiset2023). The social transactional model of psychiatric vulnerability in adults exposed to CA suggests that such aberrant facial emotion processing reflects biased threat processing which could inadvertently impact social functioning and relations, and thereby increase vulnerability for psychiatric disorders (McCrory et al., Reference McCrory, Foulkes and Viding2022). Indeed, functional imaging studies showed that resilient adults with CA show improved ability to regulate emotions through medial prefrontal cortex–limbic downregulation, lower hippocampal activation to emotional faces, and increased amygdala habituation to stress (reviewed in Moreno-López et al., Reference Moreno-López, Ioannidis, Askelund, Smith, Schueler and van Harmelen2020). It should be examined if increased nodal degree in the anterior FG aids resilient functioning through improved facial emotion processing.
Strengths of this study include a large sample of carefully assessed participants recruited from the community with low to moderate CFA experiences and the use of a MPM protocol. One limitation is that we focused on CFA limiting the generalizability of results to individuals exposed to other types of CA. Furthermore, individuals with current treatment for psychiatric disorders were excluded and the lower spectrum of CFA was overrepresented in this study. Therefore, future studies are needed to investigate whether similar or distinct mechanisms aid resilient functioning in individuals with more extreme CFA experiences. A limitation of our residual variance approach to quantify resilient functioning is that this entails a strong association between psychosocial functioning and the measures of functioning; as the residuals will, by design, be highly correlated with psychosocial outcomes. However, in our sample CFA severity was correlated significantly with psychosocial outcomes as such, our approach can explicitly separate functioning outcomes towards the extremes of CFA severity. As an example, an individual who has experienced little or no CFA will have lower resilient functioning scores than an individual who experienced severe CFA, even if the latter may have lower absolute psychosocial functioning (see Figure 1b). Another limitation of our study is that we did not include subcortical regions in our analysis. Future studies should aim to explore structural covariance between and among cortical and subcortical gray matter structures to describe a more substantive and thorough structural covariance network underlying possible differences in for instance social cognition and emotional regulation in adolescents. Also, it was beyond the scope of this study to incorporate different structural covariance measures while other measures, such as modularity, show interesting developmental effects as well (e.g., Aboud et al., Reference Aboud, Huo, Kang, Ealey, Resnick, Landman and Cutting2019). Further, the results of any sliding window method are dependent on the parameters used (i.e., window width and step size), in addition to the resilience scores being non-homogeneously distributed. However, we systematically varied these parameters, and focused on only those regions showing significant convergent results across ten parameter combinations.
CA is one of the strongest predictors of mental health problems in later life, and as such it is critical that we better understand how resilience can be achieved in young people with CA. To do so, resilience research examines why some young people with CA go on to develop mental illness, whereas others do not. By better understanding this variability in functioning outcomes in CA-exposed individuals, resilience research represents a shift away from a disease focussed model towards a preventative model. As such, resilience research aims to inform intervention and prevention efforts for individuals at risk (Masten, Reference Masten2019; Luthar & Cicchetti, Reference Luthar and Cicchetti2000). Recent models emphasize that resilience is facilitated by complex interrelations across cultural, social, psychological, and neurobiological systems and their development over time (Masten & Cicchetti, Reference Masten and Cicchetti2010; Masten et al., Reference Masten, Lucke, Nelson and Stallworthy2021). Within this multisystem framework, neuroimaging studies help inform our understanding of the neurobiological mechanisms that help aid resilient functioning (Ioannidis et al., Reference Ioannidis, Askelund, Kievit and van Harmelen2020). By studying how these mechanisms interact with social or psychological systems these studies can provide key insights for prevention or intervention efforts (Cicchetti & Toth, Reference Cicchetti and Toth2015). In this study, we integrated two systems: individual psychosocial functioning and the brain. We examined the structural network topology of resilient functioning in adolescents and young adults exposed to CFA. We found that higher resilient functioning was convergently associated with lower nodal degree of DLPFC, MPFC, PSTS, ITG and MTG, LOCC, pericalcarine cortex, and the premotor cortex. These regions all play a role in a wide array of functions, of particular interest is their role in social-emotional functioning. Developmental changes in adolescence include extensive remodeling of structural covariance patterns, including reductions in nodal degree. As previous work in this sample showed negative associations between age and nodal degree (Váša et al., Reference Váša, Seidlitz, Romero-Garcia, Whitaker, Rosenthal, Vértes, Shinn, Alexander-Bloch, Fonagy, Dolan, Jones, Goodyer, Sporns and Bullmore2018; Whitaker et al., Reference Whitaker, Vértes, Romero-Garcia, Váša, Moutoussis, Prabhu, Weiskopf, Callaghan, Wagstyl, Rittman, Tait, Ooi, Suckling, Inkster, Fonagy, Dolan, Jones, Goodyer, Goodyer and Villis2016), our findings of lower nodal degree being related to higher resilient functioning may be compatible with more mature-like structural network topology in more resilient young people with CFA.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0954579423000901.
Acknowledgements and funding sources
The authors are grateful to the volunteers who took part in these studies, as well as all the members of staff involved in the recruitment process. This work was supported by the Neuroscience in Psychiatry Network (NSPN) Consortium, a strategic award from the Wellcome Trust to the University of Cambridge and University College London (095844/Z/11/Z); the Leiden Social Resilience and Security program, the Cambridge NIHR Biomedical Research Center and by the Max Planck–UCL Center for Computational Psychiatry and Ageing, a joint initiative of the Max Planck Society and University College London; a Royal Society Dorothy Hodgkin Fellowship for Prof. Anne-Laura van Harmelen (DH150176); an NIHR Senior Investigator award for Prof. Ed Bullmore and a Wolfe Health Fellowship for Dr Laura Moreno-López. Dr František Váša was supported by the Gates Cambridge Trusts, the Data to Early Diagnosis and Precision Medicine Industrial Strategy Challenge Fund, UK Research and Innovation, and the Bill & Melinda Gates Foundation.
Author contribution
LML and NGG analyzed the imaging data and drafted the manuscript with EELB, SNS, MS and ALvH. ALvH conceptualized and designed the study, analyzed the behavioral data, and reviewed and revised the manuscript. All authors approved the final manuscript and agreed to be accountable for all aspects of the work presented.
Competing interests
ETB is a member of the scientific advisory board for Sosei Heptares and a consultant for GlaxoSmithKline.






