1 Introduction
Depression (depressive disorder [DD]) is considered to be the most common mental disorder. Estimations show that 350 million people worldwide suffer from this disease. By the year 2020, it will have become the second most common health problem in the world, only after ischaemic heart disease [1].
The aetiology of this disease has not been examined thoroughly so far and is not completely known Reference Marcus, Yasamy, Ommeren, Chisholm, Saxena, Marcus, Yasamy, Ommeren, Chisholm and Saxena[2]. However, certain evidence shows that an imbalance in the generation and elimination of reactive oxygen and nitrogen species (ROS and RNS, respectively) is present during depression Reference Maes, Galecki, Chang and Berk[3]. This imbalance leads to increased levels of biomarkers of oxidative and nitrosative process intensification, such as 8-hydroxyguanine (8-oxoG), 8-iso-prostaglandin F2α (8-izo-PGF2α), malondialdehyde (MDA), and nitric oxide (NO) [Reference Stefanescu and Ciobica4–Reference Selek, Savas, Gergerlioglu, Bulbul, Uz and Yumru8]. Interestingly, a recent study has shown that increased level of MDA is associated with a reduced ability of the visual-spatial and auditory-verbal working memory and short-term declarative memory, while a high concentration of this biomarker in depressed patients’ plasma may be positively correlated with the intensity of the symptoms Reference Michel, Pülschen and Thome[9]. Changes in the activity of antioxidant enzymes may be some of the reasons for the imbalance. Accordingly, it has been demonstrated that low activity of glutathione peroxidase (GPx, reduces hydrogen peroxide to water and reduces lipid hydroperoxides) may contribute to the development of depression Reference Maes, Mihaylova, Kubera, Uytterhoeven, Vrydags and Bosmans[10]. Moreover, the same researchers have found that GPx activity is correlated with the severity of the disease – the lower the enzyme activity is, the more severe the symptoms are. In addition, activity of the next antioxidant enzyme, i.e. glutathione reductase (GR, reduces glutathione disulphide to the sulfhydryl form of glutathione), decreases in depressed patients as compared to healthy volunteers Reference Michel, Pülschen and Thome[9]. On the other hand, increased levels and activity of catalase (CAT, reduces hydrogen peroxide to water and oxygen) may serve as a risk factor for the occurrence of depressive episodes [Reference Szuster-Ciesielska, Słlotwińnska, Stachura, Marmurowska-Michałlowska, Dubas-Slemp and Bojarska-Junak11, Reference Gałecki, Szemraj, Bieńkiewicz, Zboralski and Gałecka12]. The plasma activity of another antioxidant enzyme, i.e. superoxide dismutase (SOD, catalyses the reaction of superoxide radical dismutation into oxygen or hydrogen peroxide), also increases in the course of depression, which has been proven in various experiments conducted on animal models and during clinical studies [Reference Gałecki, Szemraj, Bieńkiewicz, Zboralski and Gałecka12, Reference Tagliari, dos Santos, Cunha, Lima, Delwing and Sitta13]. However, it has been proven that the over-activation of SOD may lead to the intensification of oxidative stress via H2O2 production Reference Michel, Pülschen and Thome[9]. A change in SOD levels has been observed in the brain tissue collected from depressed patients. An elevated level of copper/zinc (Cu/Zn) SOD has only been detected in post-mortem prefrontal cortical brain tissue, and not in the hippocampus, while the level of manganese SOD (MnSOD) has not changed in both regions of patients’ brain when compared to control subjects. Different locations of these isoforms may serve as an explanation for these differences – Cu/ZnSOD is present primarily in the cytosol of glial cells, while MnSOD is found mainly in neurons and erythrocytes. So far the available results suggest that elevated levels of SOD in peripheral tissues (plasma, erythrocytes, saliva) may be reduced owing to a successful antidepressant therapy Reference Michel, Frangou, Thiemeyer, Camara, Jecel and Nara[14]. Galecki et al. (2009) found that a combined therapy with the application of fluoxetine (SSRI) and acetylsalicylic acid may lead to a decrease in the activity of Cu/ZnSOD and a reduction in MDA concentration Reference Galecki, Szemraj, Bienkiewicz, Zboralski and Galecka[15]. In addition, antidepressants may also result in the normalisation of serum paraoxonase activity (reduced oxidation of apolipoprotein B containing lipoproteins) Reference Michel, Frangou, Thiemeyer, Camara, Jecel and Nara[14].
Low amounts of non-enzymatic antioxidants are considered another aspect associated with the risk of depression occurrence. So far, studies have shown that the women with DD have lower amounts of glutathione (GSH) than the women not affected by depression Reference Kodydková, Vávrová, Zeman, Jirák, Macásek and Stanková[16]. Moreover, a decreased concentration of GSH has been observed in the chronic mild stress animal model of the disease Reference Kumar, Kuhad and Chopra[17]. Similarly, the level of CoQ10 has been dramatically reduced in the serum of the patients suffering from DD as compared to the control group Reference Maes, Mihaylova, Kubera, Uytterhoeven, Vrydags and Bosmans[18]. Moreover, based on an animal model, it has been possible to determine that low-zinc diet reduces the number of progenitor cells and immature nerve cells in the hippocampus of treated rats Reference Suh, Won, Hamby, Yoo, Fan and Sheline[19]. Furthermore, decreased levels of yet another group of non-enzymatic antioxidants – vitamins A, C and E – may also play an important part in the aetiology of depression; however, the results are not conclusive in this respect. On the one hand, the plasma amount of ascorbic acid (vitamin C) is reduced in the patients with DD Reference Khanzode, Dakhale, Khanzode, Saoji and Palasodkar[20]. On the other hand, it has been suggested that increased plasma levels of vitamin C can be associated with the severity of DD Reference Kobrosly and van Wijngaarden[21]. Results of a different study have indicated no difference in the plasma levels of vitamins A, C and E between the patients and the control group Reference Pandya, Howell and Pillai[22]. However, Maes et al. Reference Maes, De Vos, Pioli, Demedts, Wauters and Neels[23] found that the plasma level of vitamin E of the affected patients was lower as compared to healthy volunteers. The discrepancies in the results may be due to size differences of the studied groups, the environmental impacts and the severity of the disease. Additionally, the patients with DD are also characterised by decreased levels of other non-enzymatic antioxidants such as albumin and uric acid Reference Michel, Pülschen and Thome[9].
Another piece of evidence that supports the hypothesis of ROS and RNS involvement in the pathogenesis of the disease are changes in the level and activity of oxidative and nitrosative enzymes in the patients with DD. It has been revealed that depressed patients have increased serum levels of xanthine oxidase (XO) [Reference Maes, Galecki, Chang and Berk3, Reference Herken, Gurel, Selek, Armutcu, Ozen and Bulut24]. This enzyme catalyses the oxidation of hypoxanthine to xanthine and then the oxidation of xanthine to uric acid resulting in the generation of superoxide anion and hydrogen peroxide Reference Terada, Willingham, Roasandich, Leff, Kindt and Repine[25]. The patients with depression are characterised by elevated XO activity in the thalamus, the putamen, and the frontal and parietal cortex, the hippocampus and the caudate nuclei; XO activity has been found to be decreased in the temporal and occipital cortex [Reference Michel, Pülschen and Thome9, Reference Michel, Camara, Tatschner, Frangou, Sheldrick and Riederer26]. A recent study has revealed that the main symptoms of depression – cognitive dysfunction, anhedonia and melancholia – may be associated with structural or functional neuronal changes of the putamen and the thalamus Reference Michel, Pülschen and Thome[9]. Additionally, patients with depression demonstrate increased expression of cellular NOS in the neurons of the suprachiasmatic nucleus, cornu ammonis area 1 (CA1), and subiculum regions as compared with the control group Reference Oliveira, Guimarães and Deakin[27]. A growing body of evidence suggests that the factors involved in nitrosative stress may penetrate the blood-brain barrier exhibiting their depressive and neurotoxic activities in the brain. As a result of excessive pro-oxidative enzyme activity (such as XO, NOS), the levels of ROS and RNS are increased, which may lead to the development of neurodegenerative changes [Reference Leonard and Maes28, Reference Moylan, Berk, Dean, Samuni, Williams and O’Neil29]. A large amount of ROS may induce apoptosis of neural cells by causing damage to DNA or peroxidation of the cell membrane lipid (ROS destroy the lipids of cells, mainly polyunsaturated acids [PUFAs]) Reference Michel, Pülschen and Thome[9]. This long-lasting condition may be one of the causes of death of neuronal and glial cells in the central nervous system, observed in neurodegenerative diseases Reference Michel, Camara, Tatschner, Frangou, Sheldrick and Riederer[26]. Interestingly, the study suggests that the patients with DD have a reduced volume of the prefrontal cortex and the hippocampus as compared to healthy volunteers. Furthermore, a post-mortem study confirmed that the patients with DD had a reduced number and density of glial cells Reference Michel, Frangou, Thiemeyer, Camara, Jecel and Nara[14].
Moreover, the study suggested that RNS (e.g. peroxynitrite) may cause nitration of biological compounds, including amino acids (mainly tyrosine). Additionally, Maes et al. Reference Maes, Galecki, Chang and Berk[3] have found increased levels of IgM antibodies to such modified proteins in the blood samples collected from depressed patients.
An imbalance in the production and elimination of ROS and RNS – leading to oxidative and nitrosative stress – may induce various disorders. Oxidative stress is involved in the development of cardio-vascular and neuropsychiatric disorders such as ischaemia, acute respiratory distress syndrome (ARDS), panic disorder Reference Herken, Akyol, Yilmaz, Tutkun, Savas and Ozen[30], preeclampsia Reference Karabulut, Kafkasli, Burak and Gozukara[31], autism Reference Zoroglu, Armutcu, Ozen, Gurel, Sivasli and Yetkin[32], dementia Reference Michel, Nara, Camara, Koutsilieri, Jecel JCh and Riederer[33], schizophrenia Reference Michel, Nara, Camara, Koutsilieri, Thome and Riederer[34], Parkinson's disease, Alzheimer's disease [Reference Michel, Gsell, Geuder, Frangou, Durany and Kircher35, Reference Michel, Gsell, Käsbauer, Tatschner, Sheldrick and Neuner36], dementia Reference Durany, Münch, Michel and Riederer[37], amyotrophic lateral sclerosis, schizophrenia and depression [Reference Michel, Frangou, Thiemeyer, Camara, Jecel and Nara14, Reference Michel, Nara, Camara, Koutsilieri, Jecel JCh and Riederer33, Reference Michel, Nara, Camara, Koutsilieri, Thome and Riederer34, Reference Riederer and Lange38–Reference Michel, Thome, Nara, Martin, Camara and Weijers41], and multiple sclerosis Reference Jana and Pahan[42]. Moreover, a mitochondrial dysfunction can cause overproduction of ROS and – in consequence – may lead to the development of ischaemic heart disease, stroke, atherosclerosis, arterial hypertension, and hypertrophy of the myocardium Reference Andalib, Divani, Michel, Høilund-Carlsen, Vafaee and Gjedde[43]. On the other hand, nitrosative stress is involved in the development of Parkinson's disease Reference Chung[44], Alzheimer's disease Reference Calabrese, Sultana, Scapagnini, Guagliano, Sapienza and Bella[45], schizophrenia Reference Anderson, Berk, Dodd, Bechter, Altamura and Dell’osso[46], depression Reference Maes, Galecki, Chang and Berk[47], cardiomyopathy, heart failure Reference Ungvári, Gupte, Recchia, Bátkai and Pacher[48], stroke, arthritis, multiple sclerosis, hypercholesterolemia, ischemia Reference Foster, McMahon and Stamler[49], and cancer [Reference Rasheed, Beevi, Rajaraman and Bose50–Reference Arsova-Sarafinovska, Eken, Matevska, Erdem, Sayal and Savaser52].
The aforementioned studies indicate that intensification of oxidative and nitrosative stress, caused, among others, by decreased levels and activity of enzymatic antioxidants and/or excessive pro-oxidative enzyme activity, may play an important role in depression aetiology. Therefore, the aim of this study was to investigate the association between the occurrence of SOD2, CAT, GPx4, NOS1 and NOS2 polymorphisms and the risk of depression development by means of determining the frequency of occurrence of genotypes of selected SNPs in the patients with DD as compared to healthy volunteers in the Polish population.
Table 1 shows the characteristic features of the studied polymorphisms.
Table 1 Characteristics of studied polymorphisms.

2 Materials and methods
2.1 Subjects
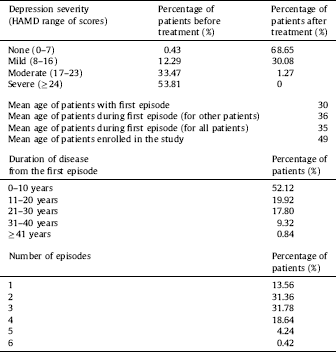
The study was conducted on 510 participants, including patients with DD (n = 281, 117 women and 114 men; mean age 53.19 ± 12.61) who were hospitalized at the Department of Adult Psychiatry of the Medical University of Lodz (Poland), and healthy controls (n = 229, 147 women and 132 men; mean age 49.53 ± 10.175). Detailed characteristics of the patients are shown in Table 2. All the participants were selected randomly without replacement sampling. Qualified patients met the diagnostic criteria for depressive episode and recurrent depressive disorder according to WHO [53]. The inclusion criteria were based on those outlined in ICD-10 (F32.0–7.32.2, F33.0–F33.8). A case history was obtained from each patient using the standardized Composite International Diagnostic Interview (CIDI) Reference Patten[54] prior to the start of the experiment. Depression severity was evaluated and classified using the 21-item Hamilton Depression Rating Scale (HDRS) Reference Hamilton[55]. Intensity levels of depressive symptoms were measured with the use of the grades presented in the study conducted by Demyttenaere and De Fruyt Reference Demyttenaere and De Fruyt[56]. Each patient was examined by the same psychiatrist (CIDI and HDRS). A psychiatric evaluation was performed before the patient was included in the study and after antidepressant therapy of selective serotonin reuptake inhibitors (SSRIs). All the subjects were examined during their hospitalisation and no symptoms of concurrent somatic diseases or axis I and II disorders, other than depressive episodes, were diagnosed in them. Inflammatory or autoimmune disorders, central nervous system traumas, and unwillingness to give informed consent were additional exclusion criteria. Patients with familial prevalence of mental disorders other than recurrent depressive disorders were excluded from the examined group. The individuals taking part in the experiment were native Poles from central Poland (not related). They were chosen for the study group at random without replacement sampling. Participation in the study was voluntary. Before making a decision to participate in the study, the subjects were informed of the purpose and assured of the voluntary nature of the experiment, and guaranteed that their personal data would be kept in secret. The patients and healthy volunteers were informed about the details of this experiment and gave their written consent to participate in this study, according to the protocol approved by the Bioethics Committee of the Medical University of Lodz (no. RNN/70/14/KE).
Table 2 The detailed characteristic of patients which were qualified the study.
2.2 Selection of single-nucleotide polymorphisms
The studied SNPs in ROS and RNS genes were selected from the database of Single Nucleotide Polymorphisms of the National Center for Biotechnology Information (NCBI dbSNP), available at http://www.ncbi.nlm.nih.gov/snp (Bethesda, MD, USA). The following six polymorphisms were chosen: c.47T > C (p.Val16Ala) (rs4880) in SOD2, c.-89A > T (rs7943316) in CAT, c.660T > C (rs713041) – GPx4, c.-420-34221G > A (rs1879417) in NOS1, c.1823C > T (p.Ser608Leu) (rs2297518), and c.-227G > C (rs10459953) in NOS2, which a minor allele frequency (MAF) higher than 0.05 in the European population (submitter population ID: HapMap-CEU). All polymorphisms are located in the coding or regulatory regions of genes and may have functional significance for transcription and protein function.
2.3 DNA extraction
Genomic DNA was isolated from venous blood using commercially available Blood Mini Kit (A&A Biotechnology, Gdynia, Poland). Blood samples were collected from each of the patients with DD before antidepressant therapy, and from all healthy volunteers. DNA purity and concentration were measured by comparing the absorbance at 260 and 280 nm; after that, the samples were stored at −20 °C until use.
2.4 Genotyping
The tested SNPs were genotyped using the TaqMan SNP Genotyping Assay (Thermo Fisher Scientific, Waltham, Massachusetts, USA) and 2X Master Mix Takyon for Probe Assay–No ROX (Eurogentec, Liège, Belgium), according to the manufacturer's instructions. Real-time PCRs were carried out in the Bio-Rad CFX96 Real-Time PCR Detection System and analysed in the CFX Manager Software (Bio-Rad Laboratories Inc., Hercules, California, USA).
2.5 Statistical analysis
A statistical analysis of the data was performed using Statistica 12 (Statsoft, Tulsa, OK, USA) and SigmaPlot 11.0 (Systat Software Inc., San Jose, CA, USA). The association between case/control and each studied polymorphism was estimated using an unconditional multiple logistic regression model. The results are shown as odds ratios (ORs) with 95% confidence interval (95% CI). In addition, the OR was adjusted for sex, since women are exposed to a doubled risk of depression in comparison to men Reference Moylan, Berk, Dean, Samuni, Williams and O’Neil[29]. The data presenting the results from the distribution of genotypes by age of the first episode of depression are indicated as the median ± inter-quartile range. The normality of distribution was verified using the Shapiro-Wilk test and then the significance of differences between the studied values was determined, accordingly, by either the Mann-Whitney test or Student's t test. The Student's t test was used only in the case of the c.1823C > T–NOS2 (rs2297518) polymorphism; the Mann-Whitney test was used for the remaining polymorphisms.
3 Result
3.1 Single nucleotide polymorphism of the SOD2, CAT, GPx4, NOS1 and NOS2 gene and depression occurrence
Table 3 shows the distribution of genotypes and alleles of the studied polymorphisms of the SOD2, CAT, GPx4, NOS1 and NOS2 gene in the patients with DD and in healthy volunteers. The distribution of the genotypes in all groups was in agreement with the Hardy-Weinberg equilibrium. The results demonstrated that the T/T genotype of the c.47T > C (rs4880) polymorphism of the SOD2 gene was associated with an increased risk of depression. In the case of c.-89A > T (rs7943316) – CAT, the A/A genotype was linked with an increased risk of DD occurrence, while the A/T genotype of the same polymorphism reduced this risk. Moreover, genotype T/T and allele T of c.660T > C (rs713041) – GPx4 increased the risk of DD development, while the T/C heterozygote and C allele diminished this risk.
Table 3 Distribution of genotypes and alleles of c.804-7C > A, c.-1668T > A, c.803 + 221C > A, c.-173A > T, c.-1449C > A and c.-844G > T and the risk of DD.

P < 0.05 along with corresponding ORs are in bold.
a OR adjusted for sex.
No correlation was found between genotypes/alleles of the c.-420-34221G > A (rs1879417)–NOS1, c.1823C > T (rs2297518), and c.-227G > C (rs10459953)–NOS2 polymorphisms, and DD development.
3.2 Single-nucleotide polymorphisms of genes encoding enzymes of oxidative and nitrosative stress, and the age of the first episode of depression and the severity classification on the Hamilton Depression Rating Scale
A difference was found in the age distribution of the first depressive episode between the T/T and T/C genotypes of the c.47T > C–SOD2 (rs4880) polymorphism. Moreover, a difference between the A/A and A/T genotypes of the c.-89A > T–CAT (rs7943316) polymorphism was detected. However, no association was found for the remaining polymorphisms studied (Fig. 1 and Supplementary Fig. 1). Moreover, no significant differences in the distribution of the severity classification on the Hamilton Depression Rating Scale and the genotypes of studied SNPs (data unpublished) were revealed.

Fig. 1 Distribution of the age of the first episode of depression and single-nucleotide polymorphisms of genes encoding SOD2 and CAT. The horizontal lines denote the median, while the whiskers show the inter-quartile range.
3.3 Single-nucleotide polymorphisms of genes encoding enzymes of oxidative and nitrosative stress, and depression occurrence in male and female population
Previous studies have suggested that women have been exposed to a doubled risk of depression development in comparison to men Reference Kessler[57]. Therefore, we decided to investigate the association between the prevalence of DD in male/female population and all studied polymorphisms (Table 4). In the case of the c.47T > C (p.Val16Ala)–SOD2 (rs4880) polymorphism, the T/T genotype and T allele increased the risk of DD in men, while the C allele of the same SNP reduced this risk in the population. However, no correlation between the studied polymorphisms and the risk of DD in female population was detected and confirmed. Furthermore, the A/A genotype of c.-89A > T–CAT (rs7943316) decreased the risk of depression in men, while the A/T and T/T genotypes of the same polymorphism reduced the risk in women. The T/T genotype and the T allele of the c.660T > C (rs713041)–GPx polymorphism are associated with the occurrence of depression in both groups studied. Moreover, the T/C heterozygote of the same SNP caused an increase of DD risk only in the male population. On the other hand, the C allele of the same polymorphism was associated with a decreased risk in the group of males and females. In the case of c.-227G > C–NOS2 (rs10459953), the G/C genotype was associated with a reduced risk of depression development in women, while the G/G genotype of the same polymorphism increased this risk in the population. However, no correlation between the polymorphism and the appearance of depressive disorders was found in the male population.
Table 4 Distribution of genotypes and alleles of c.804-7C > A, c.-1668T > A, c.803 + 221C > A, c.-173A > T, c.-1449C > A and c.-844G > T and the risk of DD in male and female population.

P < 0.05 along with corresponding ORs are in bold.
a OR adjusted for sex.
Moreover, the differences between the distribution of genotypes and alleles and the sex of the patients with depression were investigated. It was revealed that the C/C genotype of the c.660T > C–GPx4 polymorphism occurred about two times more seldom in women than in men (Crude OR [95% CI] = 0.574 [0.343–0.963], P = 0.035). This association was not found for the remaining polymorphisms studies (data not published).
3.4 Haplotypes and DD prevalence
Our team also studied the correlation between the occurrence of depressive disorders and haplotypes of the c.1823C > T (rs2297518) and c.-227G > C (rs10459953) polymorphisms of the NOS2 gene. Supplementary Table 1 shows the association between depression and haplotypes of the studied polymorphisms. No correlation between the studied haplotypes of the two polymorphisms and the risk of DD was found.
3.5 Gene-gene interactions and the risk of depression
We also investigated the correlation between the occurrence of DD and combined genotypes of the studied polymorphisms. The distribution of the combined genotypes is presented in Table 5 and Supplementary Table 2. We observed that the A/T-T/T combined genotype of the c.-89A > T (rs7943316)–CAT and c.47T > C (rs4880)–SOD2 polymorphisms was associated with an increased risk of DD. Moreover, the T/T-T/T combined genotypes of c.47T > C–SOD2 (rs4880) and c.1823C > T–NOS2 (rs2297515) caused a nearly five-fold increase of the risk in the Polish population. The link between a reduced risk of depression and the frequency of the T/C-C/G genotype of the c.47T > C (rs4880) – SOD2 and c.-227G > C (rs10459953) – NOS2 polymorphisms was confirmed in our study. Additionally, the presence of the T/C-T/T combined genotype of the c.47T > C (rs4880)–SOD2 and c.660T > C (rs713041)–GPx4 polymorphisms elevated the risk of depression development more than 11 times. On the other hand, the T/C-T/C and T/C-C/C genotypes of the same combination of polymorphisms were associated with a diminished risk of DD occurrence. The A/T-T/T and T/T-T/T genotypes of c.-89A > T (rs7943316)–CAT and c.660T > C (rs713041)–GPx4 caused an increase of the risk of DD by just about six times, whereas the A/T-T/C and T/T-C/C genotypes of the same combined polymorphisms brought about a reduction of this risk. In the case of c1823C > T (rs2297518) and c. 660T > C (rs713041), combined genotypes T/T–T/T were associated with a twelvefold increase of the DD risk. Moreover, we found that the G/A-T/T genotype of c.-420-3422G > A (rs187944)–NOS1 and c.660T > C (rs713041)–GPx4 increased the risk of depression nearly sevenfold. However, the G/C-T/T and G/G-T/T genotypes of the c.-227G > C (rs10459953)–NOS2 and c.660T > C (rs713041)–GPx4 combined polymorphisms were positively correlated with depression, while genotype G/C-C/C of the same SNP combination was negatively correlated with the disease. On the other hand, the T/T-C/T genotype of the c.-89A > T (rs7943316)–CAT and c.1823C > T (rs2297518)–NOS2 combined polymorphisms increased the risk of depression, while the T/T-T/T genotype of the same combination reduced the risk. In the case of c.-89A > T (rs7943316)–CAT and c.-227G > C (rs10459953)–NOS2, combined genotype A/T-G/G was associated with occurrence of DD. Moreover, the A/T-G/A genotype of the c.-89A > T (rs7943316)–CAT and c.-420-34221G > A (rs1879417)–NOS1 combined polymorphisms increased the risk of depression occurrence, while the A/A-A/A genotype of the same polymorphisms combination reduced this risk.
No statistical correlation was found between combined genotypes of c.47T > C (rs4880)–SOD2 and c.-420-34221G > A (rs1879417)–NOS1, c.1823C > T (rs2297518)–NOS2 and c.-420-34221G > A (rs1879417)–NOS1, c.-420-34221G > A (rs1879417)–NOS1 and c.-227G > C (rs10459953)–NOS2 SNPs and the development of depressive disorders.
3.6 Single-nucleotide polymorphisms of genes encoding oxidative and nitrosative stress enzymes, and effectiveness of depression treatment
We also studied the impact of single-nucleotide polymorphisms of genes encoding enzymes, generating ROS and NOS, on the effectiveness of antidepressant treatment with the administration of SSRI. The patients were divided into two groups–those who received a maximum of 7 points on the Hamilton Rating Scale for Depression after treatment (marked as effectiveness of antidepressant therapy), and those whose total score after treatment was more than 7 points (marked as ineffective antidepressant therapy). No impact of single-nucleotide polymorphisms of genes encoding enzymes, generating of ROS and NOS, on the effectiveness of the SSRIs therapy was found (data not shown). Moreover, we investigated the distribution of genotypes of the studied polymorphism and the percentage of the Hamilton Rating Scale for Depression (Supplementary Fig. 2), yet no difference in the percentage dispersion of the Hamilton Rating Scale for Depression between genotypes of the studied polymorphism was confirmed.
4 Discussion
Previous studies suggest that the intensification of oxidative and nitrosative stress processes may play a crucial role in depression development Reference Maes, Mihaylova, Kubera, Uytterhoeven, Vrydags and Bosmans[58]. As mentioned in the Introduction, these abnormalities may be a result of the irregular functioning of the enzymes involved in the generation and elimination of ROS and RNS, such as SOD2, CAT, GPx4, NOS1 and NOS2. This is the first study to show that the chosen SNPs of genes encoding these proteins may modulate the risk of DD.
One of the most effective intracellular antioxidants is manganese superoxide dismutase (encoded by the SOD2 gene which is located on chromosome 6q25), which is a key mitochondrial enzyme and protects the cell against ROS Reference Fukai and Ushio Fukai[59]. In our study, we found the association between depression development and the occurrence of c.47T > C SNP (rs4880)–SOD2. The studied SNP brings about an transformation of amino acid from valine (Val) to alanine (Ala) at position 16, which – as a result – leads to a conformational change in the target sequence of SOD2 and decreases its antioxidant potential in mitochondria [Reference Robbins and Zhao60, Reference Valko, Rhodes, Moncol, Izakovic and Mazur61]. Moreover, it has been suggested that the T allele may be associated with lower enzymatic efficiency and the risk of higher ROS levels Reference Sutton, Khoury, Prip-Buus, Cepanec, Pessayre and Degoul[62]. In addition, another study confirmed that the Val variant may induce a 30 to 40% increase in SOD2 activity in mitochondria Reference Fujimoto, Taguchi, Imai, Ayabe, Hashimoto and Kobayashi[63]. Accordingly, our results suggest that the T/T homozygotes increase the risk of DD (Table 3). However, this genotype is associated with the development of depression only in Polish men (Table 4). Such results may reflect the differences between sexes in the regulation of enzymatic activity. So far, the polymorphism has been studied in somatic diseases and a previous study revealed that the T/T genotype was positively correlated with the occurrence of migraine symptoms in Caucasian population Reference Palmirotta, Barbanti, De Marchis, Egeo, Aurilia and Fofi[64]. Another study demonstrated that the T/T and T/C genotypes were correlated with the development of medulloblastoma in children Reference Brackett, Krull, Scheurer, Liu, Srivastava and Stovall[65].
Table 5 Distribution of the combined genotype of the studied polymorphisms and risk of the depression.


P < 0.05 along with corresponding ORs are in bold.
a OR adjusted for sex.
The next important antioxidant enzyme, which converts hydrogen peroxide into water and oxygen, is CAT. Its gene is located on chromosome 11p13, while the studied c.89A > T polymorphism (rs7943316) of the gene is present in its promoter region and may cause a decrease of its expression and enzyme activity Reference Park, Ha, Uhm, Jin, Kim and Chung[66]. So far, the CAT polymorphism has not been studied in mental disorders, but it has been shown that the T/T genotype and T allele are more frequent in the patients with vitiligo as compared to controls in the population of Gujarat Reference Mansuri, Jadeja, Singh, Laddha, Dwivedi and Begum[67]. Similarly, we found that the A/A genotype of the polymorphism was associated with a decreased risk of depression development in Polish population, while the A/T genotype elevated this risk (Table 3). However, we found that the A/T and T/T genotypes were linked with a reduced risk of depression development in females, while the A/A genotype was associated with a decreased risk in males (Table 4). Such a discrepancy may be a result of differences in the regulation of enzymatic activity between men and women.
The next studied gene, GPx4, is located on chromosome 19p13.3 and encodes selenoprotein, which catalyses the reduction of hydrogen peroxide, organic hydroperoxide, and lipid peroxides by means of glutathione oxidation [Reference Ratnasinghe, Tangrea, Andersen, Barrett, Virtamo and Taylor68–Reference Brielmeier, Bechet, Suppmann, Conrad, Laux and Bornkamm70]. We were the first to detect that polymorphism c.660T > C (rs713041) of GPx4 may modulate the risk of depression development. The studied SNP is located near the insertion sequence element in the gene's 3′ untranslated region (3′UTR) and alters the protein-binding site to this region. Studies suggest that the C variant determines higher expression of GPx and protein activity than the T variant [Reference Bermano, Pagmantidis, Holloway, Kadri, Mowat and Shiel71, Reference Meplan, Crosley, Nicol, Horgan, Mathers and Arthur72]. Accordingly, we found that the T/T homozygote increased the risk of DD (Table 3). In view of that, we demonstrated that the C/C homozygote was less common in women with DD than in depressed men (Table 4.). Similar results were obtained in the case of the patients with hypertension; the T/T genotype of the studied GPx4 SNP was associated with the occurrence of cerebral stroke in the patients with essential hypertension Reference Polonikov, Vialykh, Churnosov, Illig, Freidin and Vasil’eva[73]. On the other hand, it was indicated that the heterozygote was associated with an increased risk of death in the patients with breast cancer, and correlated with the occurrence of colorectal cancer [Reference Udler, Maia, Cebrian, Brown, Greenberg and Shah74, Reference Méplan, Hughes, Pardini, Naccarati, Soucek and Vodickova75]; our results indicate the T/C carriers reduce the risk of DD.
The next three polymorphisms studied are located in the gene encoding nitric oxide synthase which catalyses conversion of L-arginine to nitric oxide. So far, three isoforms of this enzyme have been described, i.e. the neuronal type I (NOS1) and the endothelial type (eNOS), which are constitutively expressed and regulated by calmodulin and Ca2+, and the inducible type (iNOS or NOS2). NOS1 generates NO in the nervous tissue, while iNOS produces large quantities of this compound upon stimulation by proinflammatory cytokines [Reference Maes, Galecki, Chang and Berk3, Reference Di Meo, Reed, Venditti and Victor76]. Literature suggests that immune system deregulation, including abnormal levels of interleukin 1beta (IL)-1β, interleukin 6 (IL-6), interleukin 11 (IL-11), tumour necrosis factor-alpha (TNF-α), and C-reactive protein (CRP), may increase the production of NO and, as a consequence, may lead to the development of DD Reference Bufalino, Hepgul, Aguglia and Pariante[77]. Additionally, animal studies confirmed that administration of IL-1β may cause behavioural alterations and the occurrence of symptoms similar to those observed in major depression, such as anhedonia, anorexia, weight loss, social withdrawal, psychomotor retardation, irritability, and sleep disturbances. On the other hand, increased levels of regulatory T cells (CD4(+)CD25(hi) Tregs) during an antidepressant therapy can be the reason for a decrease in cytokine production and recovery from depression. Thus, regulatory T cells can indirectly reduce the production of reactive nitrogen forms and may improve mental health.
NOS1 is located on chromosome 12q24 Reference Montesanto, Crocco, Tallaro, Pisani, Mazzei and Mari[78], while NOS2 is located at 17q11.2–q12 Reference Kleinert, Schwarz and Forstermann[79]. The previous study showed that c.-420-34221G > A–NOS1 and c.1823C > T–NOS2 were linked with the longevity phenotype. Moreover, the C allele of the former SNP was associated with weakened cognitive performance in geriatric patients. In addition, this allele was associated with a lower probability of survival until very old age Reference Bufalino, Hepgul, Aguglia and Pariante[77]. The latter polymorphism is located at exon 16 of NOS2, which partly encodes the reductase domain Reference Qidwai and Jamal[80], and thus may alter the structure or function of NOS2 (F-SNP database). We were the first to study the association between NOS1 and NOS2 polymorphisms and the development of DD. No correlation between these three studied polymorphisms of genes encoding NOS and the risk of depression was found in this paper (Table 3). On the other hand, the gene-gene analysis demonstrated that genotypes of combined SNPs may strongly modulate the risk of DD occurrence (Table 5). Moreover, we confirmed that the G/C and G/G genotypes of the c.-227G > C–NOS2 (rs10459953) polymorphism were associated with the development of depression in Polish women, while no such association was found for the male population (Table 4). So far, it has been shown that NOS2 SNP is not correlated with the male infertility risk in Chinese population Reference Jiang, Duan, Shang, Wang, Gao and Tian[81]. However, the T allele of the same polymorphism increases the risk of nephritis in Henoch-Schönlein purpura children Reference Jiang, Duan, Shang, Wang, Gao and Tian[81]. Moreover, the C/C genotype and the C allele bring about an increased risk of recurrent aphthous stomatitis, whereas such a correlation has not been found in the case of the c.-227G > C polymorphism Reference Karasneh, Darwazeh, Hassan and Thornhill[82]. On the other hand, the T/T genotype of c.1823C > T SNP increases the risk of benign prostatic hyperplasia in Korean men, while c.-227G > C does not modulate this risk Reference Yoo, Kim, Chung and Chang[83]. However, a recent study has shown that the c.-227G > C polymorphism of NOS2 is linked with susceptibility to Type 2 Diabetes Mellitus and Diabetic Nephropathy in the Chinese Han population Reference Chen, Li, Yang, Zhong and Zhuang[84].
5 Conclusion
We have demonstrated that the selected SNPs of the genes involved in oxidative and nitrosative stress may have an impact on the risk of developing depressive disorders. We have found that the studied SNPs of the SOD2 and GPx4 gene may modulate depression occurrence. Therefore, these polymorphisms may be considered an independent marker of depression. Our study supports the hypothesis that oxidative and nitrosative stress may be involved in the development of depression.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgements
This work was supported with funding from a scientific research grant of the National Science Centre in Poland (no. UMO-2015/19/BNZ7/00410).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.eurpsy.2017.10.012.








Comments
No Comments have been published for this article.