Introduction
The estimation of the prevalence of the hepatitis C virus (HCV) in the general population is considered one of the 10 core indicators in the evaluation plan of the World Health Organization (WHO) to eliminate hepatitis by 2030 (World Health Organization, 2016). WHO defines HCV prevalence as the number and proportion of people living with a chronic HCV infection (HCV RNA positive or HCV antigen positive). In Belgium, the existing data on HCV prevalence focus on specific subpopulations (Van Baelen et al., Reference Van Baelen, Plettinckx, Antoine and Gremeaux2020), are outdated (Beutels et al., Reference Beutels, Van Damme, Aelvoet, Desmyter, Dondeyne, Goilav, Mak, Muylle, Pierard, Stroobant, Van Loock, Waumans and Vranckx1997), limited to specific geographic areas (Beutels et al., Reference Beutels, Van Damme, Aelvoet, Desmyter, Dondeyne, Goilav, Mak, Muylle, Pierard, Stroobant, Van Loock, Waumans and Vranckx1997), or not representative because of the sampling method (Litzroth et al., Reference Litzroth, Suin, Wyndham-Thomas, Quoilin, Muyldermans, Vanwolleghem, Kabamba-Mukadi, Verburgh, Jacques, Van Gucht and Hutse2019). In 2019, a meta-analysis of HCV prevalence studies performed between 2013 and 2019 estimated the HCV seroprevalence at 1.01% (95% CI 0.64–1.42%) and the viremic prevalence at 0.33% (95% CI 0.21–0.47%) (Muyldermans et al., Reference Muyldermans, Bielen, Botterman, Bourgeois, Colle, Deressa, Devolder, Horsmans, Hutse, Lanthier, Lasser, Platteau, Robaeys, Suin, Verhelst, Vlierberghe and Baelen2019). In the European Union/European Economic Area (EU/EEA), anti-HCV prevalence in the general population is estimated at 1.1% (95% CI 0.9–1.4), ranging from 0.1% in Belgium, Ireland, and the Netherlands to 5.9% in Italy (Hofstraat et al., Reference Hofstraat, Falla, Duffell, Hahné, Amato-Gauci, Veldhuijzen and Tavoschi2017).
Methods
The current HCV estimation was part of the first Belgian Health Examination Survey (BELHES), which was organized as a second stage of the sixth Belgian Health Interview Survey (BHIS). The BHIS was a cross-sectional epidemiological study in 2018 focusing on the health status, health behavior, and health consumption of the general Belgian population (Demarest et al., Reference Demarest, Van der Heyden, Charafeddine, Drieskens, Gisle and Tafforeau2013). The sampling frame consisted of all households listed in the National Registry and was selected through a multistage stratified sampling (Demarest et al., Reference Demarest, Van der Heyden, Charafeddine, Drieskens, Gisle and Tafforeau2013). Participants to the BHIS were invited to participate also in the BELHES. The study design of the BELHES is described more in detail elsewhere (Nguyen et al., Reference Nguyen, Hautekiet, Berete, Braekman, Charafeddine, Demarest, Drieskens, Gisle, Hermans, Tafforeau and Van der Heyden2020) and follows largely the technical protocol for hepatitis C prevalence surveys in the general population, as developed by the European Centre for Disease Prevention and Control (2020). In short, the BELHES was open to all persons who participated in the BHIS except for minors (< 18 years), BHIS participants for whom a proxy interview was conducted, and residents of the German Community. Due to limited resources, the BELHES sample was initially limited to 1,100 respondents. As for the anti-HCV estimate, this was considered to be large enough: with a power of 80%, an expected chronic HCV prevalence of 1.01% (Muyldermans et al., Reference Muyldermans, Bielen, Botterman, Bourgeois, Colle, Deressa, Devolder, Horsmans, Hutse, Lanthier, Lasser, Platteau, Robaeys, Suin, Verhelst, Vlierberghe and Baelen2019) and a lower alternative prevalence of 0.1% (Hofstraat et al., Reference Hofstraat, Falla, Duffell, Hahné, Amato-Gauci, Veldhuijzen and Tavoschi2017), a design effect of 1.3 and a 95% confidence level, a sample of 779 would have been sufficient (European Centre for Disease Prevention and Control, 2020). Recruitment stopped when regional quota was reached (Nguyen et al., Reference Nguyen, Hautekiet, Berete, Braekman, Charafeddine, Demarest, Drieskens, Gisle, Hermans, Tafforeau and Van der Heyden2020). Apart from a short face-to-face interview and a clinical examination, also blood and urine samples were collected. Exclusion criteria for blood samples included pregnancy, clotting disorders, the use of anticoagulant medicines, and a history of epileptic fits. Depending on the consent to have blood stored in a biobank or not, five or seven blood samples were taken from each respondent. Due to budgetary constraints, some analyses could not immediately be done after collection and samples were temporarily frozen at −80°C. This was the case for the analysis of HCV-antibodies which was done 10 months after the end of the fieldwork. Results were linked to the data from the BHIS and the BELHES questionnaire to determine risk factors of a positive HCV test result, such as country of birth and history of drug use (Nguyen et al., Reference Nguyen, Hautekiet, Berete, Braekman, Charafeddine, Demarest, Drieskens, Gisle, Hermans, Tafforeau and Van der Heyden2020). For key variables such as sociodemographic data, BELHES participants were compared to the other BHIS participants as well as to the general population. We applied survey weights to all results, taking into account the multistage stratified survey design, and will further report only weighted percentages, with corresponding 95% CIs if relevant. Data analysis was done using Statistical Analysis System software version 9.4 (SAS Institute Inc., Cary, NC).
The presence of HCV-antibodies was determined using an HCV quantitative nucleic acid test on the frozen blood sample, based on a proprietary dual-probe assay design (cobas; Roche Diagnostics, Basel, Switzerland). Respondents with a positive result were tested again on a fresh whole blood sample. In case of a confirmation of the positive result, an HCV RNA test through a polymerase chain reaction (PCR) (Xpert HCV Viral Load with GeneXpert; Cepheid AB, Solna, Sweden) was done.
Results
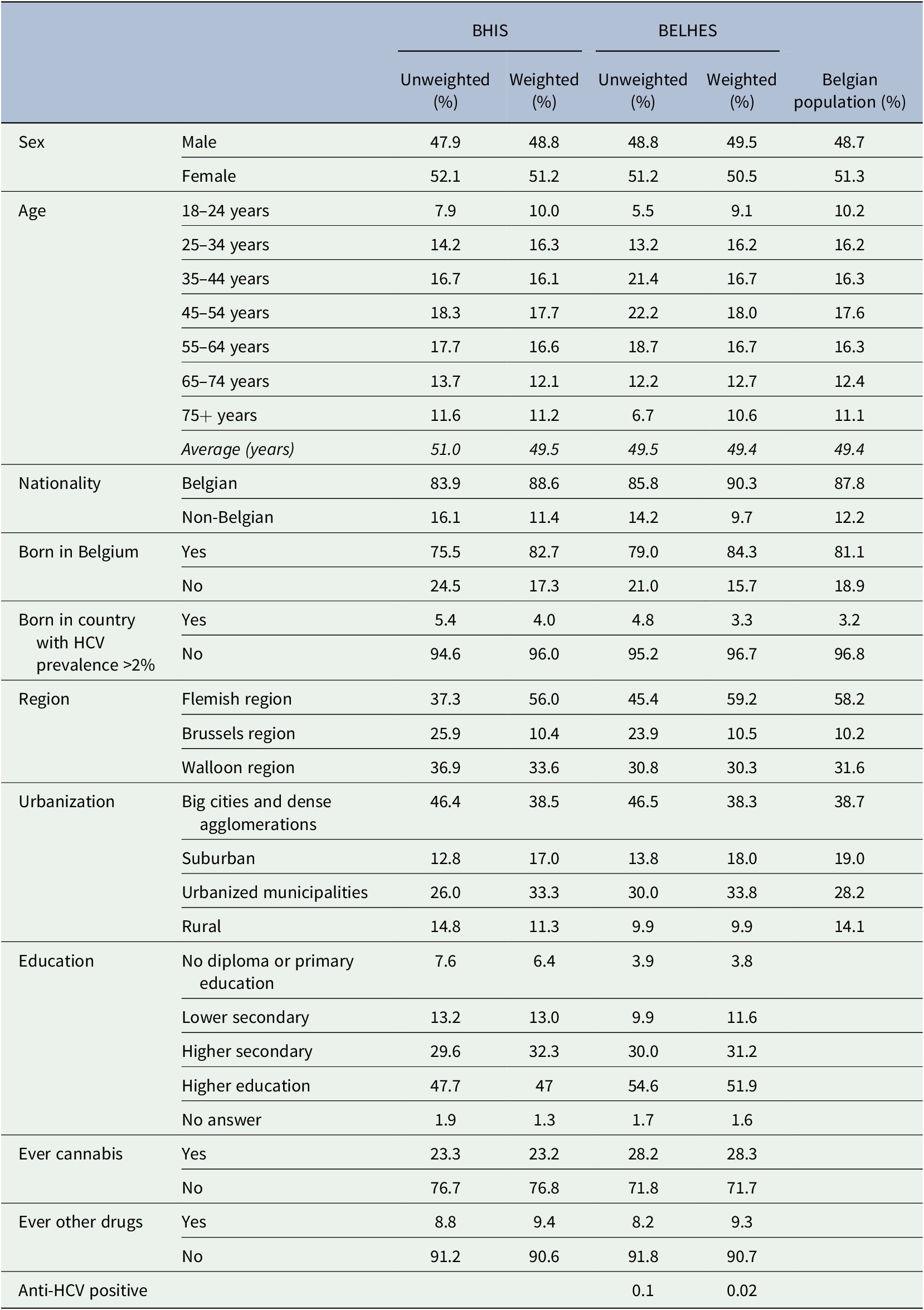
The BELHES sample consisted of 1,184 participants, which corresponds to a participation rate of 24.1% of those who were eligible in the BHIS sample and who were contacted for participation (Nguyen et al., Reference Nguyen, Hautekiet, Berete, Braekman, Charafeddine, Demarest, Drieskens, Gisle, Hermans, Tafforeau and Van der Heyden2020). For 170 BELHES participants (14.4%) blood collection was not possible due to selective refusal and difficulty in finding a vein. The remaining 1,014 respondents were tested for HCV-antibodies. As shown in Table 1, participants who were tested for HCV were mainly female (50.5%), on average of 49.4 years old, 28.3% had ever used cannabis, and 9.3% had ever used other drugs (0.1% heroin, 6.7% cocaine, 0.2% crack, and 3.5% amphetamines).
Table 1. Descriptive statistics of BHIS and BELHES samples (≥18 years), weighted and unweighted percentages, and comparison with the Belgian population where possible (Belgian Statistical Institute, n.d.)
Abbreviations: BELHES, Belgian Health Examination Survey; BHIS, Belgian Health Interview Survey.
Moreover, 90.3% had a Belgian nationality, 15.7% were not born in Belgium, and 3.3% were born in a country with HCV prevalence figures above 2% (Gower et al., Reference Gower, Estes, Blach, Razavi-Shearer and Razavi2014). Based on national data for 2018 from the Belgian Statistical Office (Belgian Statistical Institute, n.d.), people born in a country with HCV prevalence figures above 2% represent 3.2% of the Belgian population. For certain countries with a high HCV prevalence such as Gabon, Georgia, or Uzbekistan, there is only a small number of people in Belgium. The only country with high endemic HCV which was underrepresented in the study was Egypt, with only one participant in the BHIS, who did not participate in the BELHES.
BELHES respondents were higher educated than the BHIS respondents (51.9 vs. 47.0% had finished higher education), they were more living in Flanders (59.2 vs. 56.0%), they were less living in rural areas (9.9 vs. 11.3%).
Out of 1,014 samples, only two samples tested anti-HCV positive, of which one sample turned out to be false positive after the confirmation test. The other anti-HCV positive case was a woman, aged 55 years old, without known risk profile regarding drug use or migration status. Her subsequent new blood sample did not have detectable HCV RNA. Taking into account survey design settings the one positive case that was found yielded an unweighted prevalence rate of 0.1% (95% CI 0.00–0.29%) and an estimated weighted prevalence rate of 0.02% (95% CI 0.00–0.07%).
Since we only had one positive anti-HCV case, extreme cautiousness is needed to interpret the seroprevalence estimate for the general population that was obtained in this study. However, the results might indicate that the prevalence in the Belgian population is much lower than in previous estimations (Beutels et al., Reference Beutels, Van Damme, Aelvoet, Desmyter, Dondeyne, Goilav, Mak, Muylle, Pierard, Stroobant, Van Loock, Waumans and Vranckx1997; Litzroth et al., Reference Litzroth, Suin, Wyndham-Thomas, Quoilin, Muyldermans, Vanwolleghem, Kabamba-Mukadi, Verburgh, Jacques, Van Gucht and Hutse2019; Muyldermans et al., Reference Muyldermans, Bielen, Botterman, Bourgeois, Colle, Deressa, Devolder, Horsmans, Hutse, Lanthier, Lasser, Platteau, Robaeys, Suin, Verhelst, Vlierberghe and Baelen2019). The reasons for this remain unclear. Indeed, the proportion of respondents from countries with a known high HCV prevalence was representative of the general population, with the exception of respondents from Egypt. Particularly respondents from countries such as the Democratic Republic of Congo (n = 13), Romania (n = 9), or Russia (n = 4) were well represented. Moreover, based on self-reported data from the questionnaire the positive case seemed atypical. The respondent never used any illicit substances which could have been injected and she was not born in a country where HCV is highly endemic. Other information about possible risk behavior was missing.
Several limitations can be mentioned. Indeed, although there is reason to believe that, as a result of many prevention and harm reduction initiatives, the prevalence of HCV has dropped over the past decade, the low prevalence figure could also be the result of selection bias. Firstly, previous analysis has already shown that participation in the BHIS is lower for people with lower education (Demarest et al., Reference Demarest, Van der Heyden, Charafeddine, Tafforeau, Oyen and Van Hal2012). On top of that, in the current study respondents with higher education were oversampled in BELHES (51.9 compared to 47.0% for BHIS). As higher education is known to be a protective factor for HCV infection (Dalgard et al., Reference Dalgard, Jeansson, Skaug, Raknerud and Bell2003), this could have influenced our results.
Secondly, although for other known sociodemographic indicators the population structure of BELHES corresponds to the structure of the general population, some HCV risk factors were not registered in BELHES, such as history in prison, history of injecting drug use, or having tattoos. This could mean that certain hard-to-reach subpopulations such as former inmates or injecting drug users were underrepresented in BELHES. Also, the proportion of people who might be at risk for sexually transmitted infection of HCV, for example, HIV-positive men who have sex with men, is unknown in the sample. Further prevalence studies on HCV through population surveys should guarantee the presence of these subpopulations.
Finally, if the HCV seroprevalence in the general population indeed is as low as suggested by the current study, a health examination survey might not be the most appropriate research design to correctly estimate a population-wide HCV estimate. Further research should balance cost-effectiveness with the possibility to reach out for potential subpopulations at risk, while guaranteeing stochastic variation.
Data Availability Statement
Aggregate data may be made available through a formal request to the first author, who will make any and all decisions regarding data sharing. No personally identifiable data will be provided.
Competing interests
The authors declare none.
Funding statement
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Authorship Contributions
L.V.B. initiated the study and wrote the first draft of the manuscript. J.V.D.H. supervised the fieldwork. Both authors read and approved the manuscript.
Ethics Statement
The study was part of the BELHES project which was approved by the Ethical Committee of Ghent University. Participants signed an informed consent before the start of the interview.



Comments
Comments to the Author: Acronyms at first mention in the text were not written in full for e.g those found in lines 38 and 76. Kindly fix them.
While authors provided reagent details for anti-HCV testing, they failed to do the same for PCR testing. Such details are important for readership and reproducibility.
The critical study outcome which is the seroprevalence of HCV was not presented in Table 1 or any other table or figure, hence, it is difficult to link the result discussed to those presented in Table 1.
Readership will benefit immensely if authors provide statements on the potential limitations of their study, as this was not stated.