Introduction
There are at least 100 billion planets in our Galaxy alone (Cassan et al. Reference Cassan, Kubas, Beaulieu, Dominik, Horne, Greenhill, Wambsganss, Menzies, Williams, Jørgensen, Udalski, Bennett, Albrow, Batista, Brillant, Caldwell, Cole, Coutures, Cook, Dieters, Prester, Donatowicz, Fouqué, Hill, Kains, Kane, Marquette, Martin, Pollard, Sahu, Vinter, Warren, Watson, Zub, Sumi, Szymanski, Kubiak, Poleski, Soszynski, Ulaczyk, Pietrzynski and Wyrzykowski2012), and at least 20% of them are likely to fall in the habitable zone (Petigura et al. Reference Petigura, Howard and Marcy2013), the region of space capable of producing a biosphere. Even if 0.001% of those planets evolved life, that would mean 200 000 life-harbouring planets in our Galaxy; and it would only take one alien life form for our conception of the Universe to change dramatically. It is no wonder, then, that hundreds of millions of dollars have recently been invested in astrobiology research (Schneider Reference Schneider2016), the USA and Europe have rapidly growing astrobiology initiatives (Des Marais et al. Reference Des Marais, Nuth, Allamandola, Boss, Farmer, Hoehler, Jakosky, Meadows, Pohorille, Runnegar and Spormann2008; Horneck et al. Reference Horneck, Walter, Westall, Grenfell, Martin, Gomez, Leuko, Lee, Onofri, Tsiganis, Saladino, Pilat-Lohinger, Palomba, Harrison, Rull, Muller, Strazzulla, Brucato, Rettberg and Capria2016), and myriad new work has been done to try and predict what aliens will be like (Benner Reference Benner2003; Davies et al. Reference Davies, Benner, Cleland, Lineweaver, McKay and Wolfe-Simon2009; Rothschild Reference Rothschild2009; Rothschild Reference Rothschild2010; Shostak Reference Shostak2015). The challenge, however, is that when trying to predict the nature of aliens, we have only one sample – Earth – from which to extrapolate. As a result, making these predictions is hard.
So far, the main approach to making predictions about extra-terrestrial life has been relatively mechanistic (Domagal-Goldman et al. Reference Domagal-Goldman, Wright, Adamala, Arina de la Rubia, Bond, Dartnell, Goldman, Lynch, Naud, Paulino-Lima, Singer, Walter-Antonio, Abrevaya, Anderson, Arney, Atri, Azúa-Bustos, Bowman, Brazelton, Brennecka, Carns, Chopra, Colangelo-Lillis, Crockett, DeMarines, Frank, Frantz, de la Fuente, Galante, Glass, Gleeson, Glein, Goldblatt, Horak, Horodyskyj, Kaçar, Kereszturi, Knowles, Mayeur, McGlynn, Miguel, Montgomery, Neish, Noack, Rugheimer, Stüeken, Tamez-Hidalgo, Imari Walker and Wong2016). We have used observations about how things have happened on the Earth to make statistical statements about how likely they are to have happened elsewhere. For example, certain traits have evolved many times on the Earth, and so we posit that extraterrestrial life forms will converge on the same earthly mechanisms. Because eye-like organs have evolved at least 40 times (von Salvini-Plawen & Mayr Reference von Salvini-Plawen and Mayr1977), and are relatively ubiquitous, we predict that they would evolve on other planets, too (Conway Morris Reference Morris2003; Flores Martinez Reference Flores Martinez2014). Similarly, we have used a mechanistic understanding of chemistry and physics to make predictions about what is most probable on other planets. For example, carbon is abundant in the Universe, chemically versatile, and found in the interstellar medium, so alien life forms are likely to be carbon-based (Cohen & Stewart Reference Cohen and Stewart2001). These kinds of predictions come from a mixture of mechanistic understanding and extrapolating from what has happened on the Earth. There is no theoretical reason why aliens could not be silicon-based and eyeless.
An alternative approach is to use theory. When making predictions about life on other planets, a natural theory to use would be evolutionary theory. Evolutionary theory has been used to explain a wide range of features of life on the Earth, from behaviour to morphology. For example, it has allowed us to predict when some organisms, especially insects, should manipulate the sex of their offspring, to produce an excess of sons or daughters, how some birds should forage for food, and why males tend to be larger than females (Darwin Reference Darwin and Darwin1871; Clutton-Brock & Harvey Reference Clutton-Brock, Harvey and Rudder1977; Davies & Houston Reference Davies and Houston1981; West Reference West2009; Davies et al. Reference Davies, Krebs and West2012). If life arises on other planets, then the evolutionary theory should be able to make similar predictions about it. Neither approach – theoretical or mechanistic – is more or less valid than the other. But each has different advantages and can be used to make different sorts of predictions.
Here, we examine how theoretical and mechanistic approaches can be combined to better understand what to expect from alien life. We consider whether aliens will undergo natural selection, and what implications would follow if they do. That aliens undergo natural selection is something often taken for granted, but which needs justification on firm theoretical grounds. We then turn our attention to a specific subset of aliens: complex ones. We examine how complexity has arisen on the Earth, and make predictions about how complexity would arise elsewhere in the Universe. Finally, we describe some biological features we would expect to find in complex extraterrestrial life.
Natural selection
On Earth
Darwin (Reference Darwin1859) showed that just a few simple features of life on Earth lead to evolutionary change via natural selection. Individual organisms differ in how they look and act – there is natural variation. These differences are heritable – offspring tend to look and act like their parents. These heritable differences are linked to differential success – some individuals, as a result of how they are made or behave, leave more offspring than others. These three features, with heritable variation leading to differential success, result in natural selection (Darwin Reference Darwin1859; Fisher Reference Fisher1930). Any traits or behaviours linked to the greater production of offspring (higher fitness or success) will build up in the population over time. As the environment changes, different traits lead to higher success. This leads to changes in the population or evolutionary change.
Thus, the ingredients required for natural selection are incredibly simple. Given a collection of entities (a population) that has: (1) heredity; (2) variation; and (3) differential success linked to variation, then natural selection will follow. The entities that are more successful will become more prevalent in the population, as a result of being ‘selected’. Natural selection does not depend on a specific genetic system (Darwin knew nothing of modern genetics) or a specific genetic material, elemental makeup or planet-type. Given that 1, 2 and 3 exist, natural selection occurs (Fig. 1).
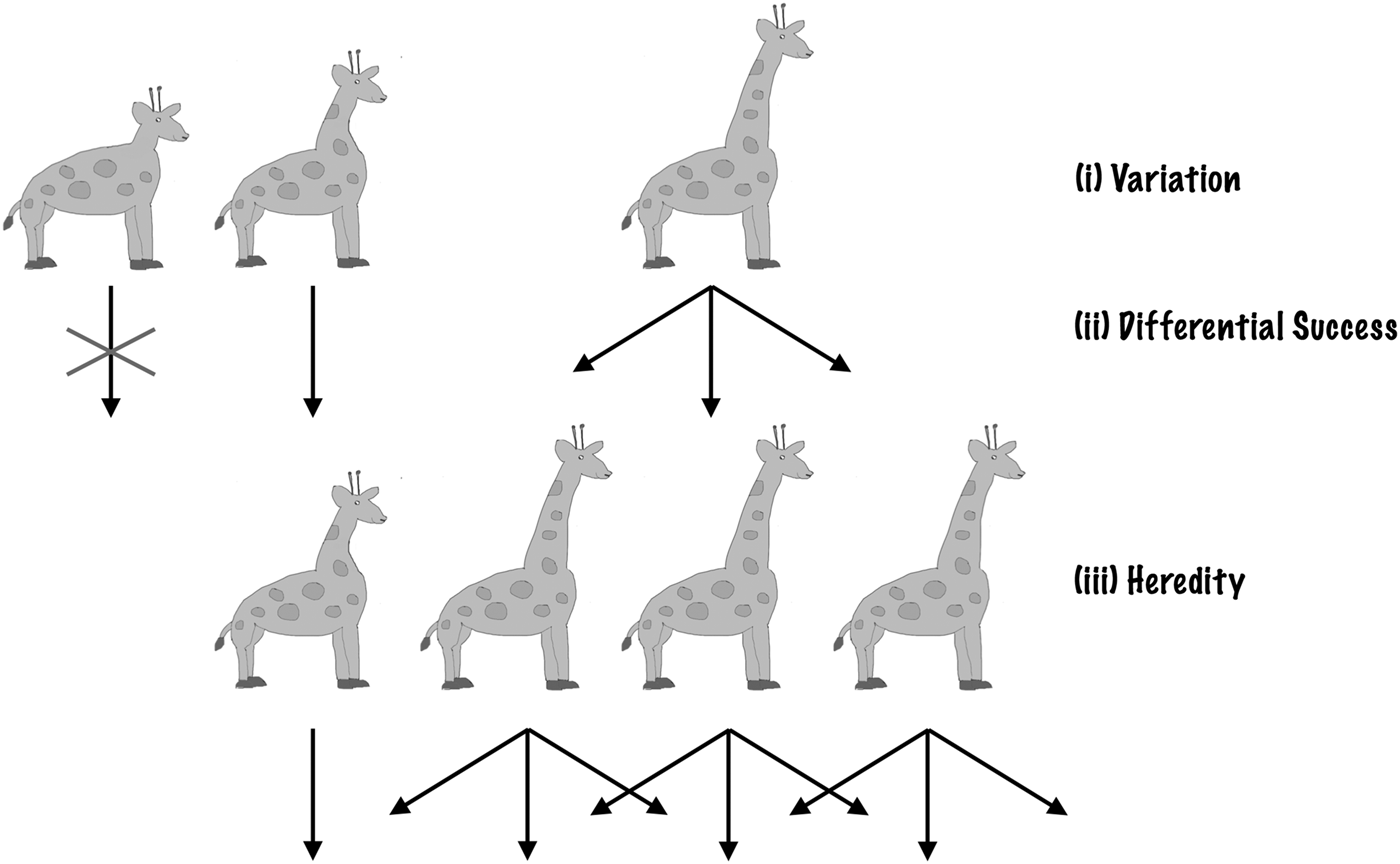
Fig. 1. Natural selection. Natural Selection operates if three conditions are satisfied: variation, differential success linked to variation and heredity. Here, we illustrate with an example: the evolution of long necks in giraffes. (i) Initially, there are natural variations in giraffes’ neck lengths. (ii) Longer-necked giraffes have access to more food, high up in the trees and so live longer to have more offspring. (iii) Giraffes’ offspring resemble their parents. As a result of (i), (ii) and (iii), the population gradually shifts to be dominated by long-necked giraffes.
Natural selection not only explains evolutionary change, it also explains adaptation. When we look around at the natural world, we cannot help but see what looks like design: a giraffe's neck is for reaching high up leaves, a stick insect's body for camouflage, a tree's leaf for photosynthesizing. Organisms look designed or ‘adapted’ for the world in which they live. Through the gradual selection of small improvements, traits associated with success in the environment accrue in the population. Consequently, over time, natural selection will lead to organisms that appear as if they were designed for success in the environment. The clause ‘as if’ is key here – natural selection leads to the appearance of design (adaptation), without a designer (Grafen Reference Grafen2003; Gardner Reference Gardner2009).
In fact, natural selection is the only explanation we have for the appearance of design without a designer (Gardner Reference Gardner2009). Other processes can cause evolutionary change. For example, a mutation can cause a change from one generation to the next. But, without natural selection, random mutation is incredibly unlikely to produce the complex traits that we see around us, like limbs or eyes. Things that appear purposeful, such as limbs, organs and cells, require the gradual selection of improvements.
Another way to say this is that natural selection is unique because it is a directional force. The entities that increase in representation in the population are a specific subset of the population – those that are better at replicating. Natural selection increases fitness (Fisher Reference Fisher1930). As a result of these ‘successful’ entities accruing in the population, over time entities become adapted for the apparent purpose of success. They look like ‘well-designed’ machines, with the ‘purpose’ of their ‘design’ being successful replication.
In space
Natural selection is the only way we know to get the kinds of life forms we are familiar with, from viruses to trees. By familiar, we are not restricting ourselves to life forms that look earthly. Instead, they are familiarly life-like in the sense that they stand out from the background of rocks and gases because they appear to be busy trying to replicate themselves. A simple replicator could arise on another planet. But without natural selection, it won't acquire apparently purposeful traits like metabolism, movement or senses. It won't be able to adapt to its environment, and in the process, become a more complex, noticeable and interesting thing.
We can ask, then, will aliens undergo natural selection? Evolutionary theory tells us that, for all but the most transient and simple molecules, the answer is yes. Without a designer, the only way to get something with the apparent purpose of replicating itself (something like a cell or a virus), is through natural selection. Consequently, if we are able to notice it as life, then it will have undergone natural selection (or have been designed by something that itself underwent natural selection).
It is easy to quibble about the definition of life, and as some authors have pointed out, trying to do so can reveal more about human language than about the external world (Cleland & Chyba Reference Cleland and Chyba2002). Our goal here is not to thoroughly define life. We adopt a functional stance – what separates life from non-life is its apparent purposiveness, leading to tasks such as replication and metabolism (Maynard Smith & Szathmáry Reference Smith and Szathmáry1995). Further, without natural selection, entities cannot adapt to their environment, and are therefore transient and will not be discovered. If we identified an extra-terrestrial entity that we deemed to be a foreign life form, but that had no degree of adaptedness, this prediction would not hold.
Picture an alien (Fig. 2). If what you are picturing is a simple replicating molecule, then this ‘alien’ might not undergo natural selection (Fig. 2a). For example, it could replicate itself perfectly every time, and thus there would be no variation, and it would never improve. Or it might have such a high error rate in replication that it quickly deteriorates. If we count things like that as life, then there could be aliens that do not undergo natural selection. But if you are picturing anything more complex or purposeful than a simple molecule, then the alien you are picturing has undergone natural selection (Fig. 2b). This is the kind of prediction that theory can make. Given heredity, variation and differential success, aliens will undergo natural selection. Or, more interestingly, without those three things, aliens could not be more complicated than a replicating molecule. Given an adapted alien, one with an appearance of design or purpose, it will have undergone natural selection.
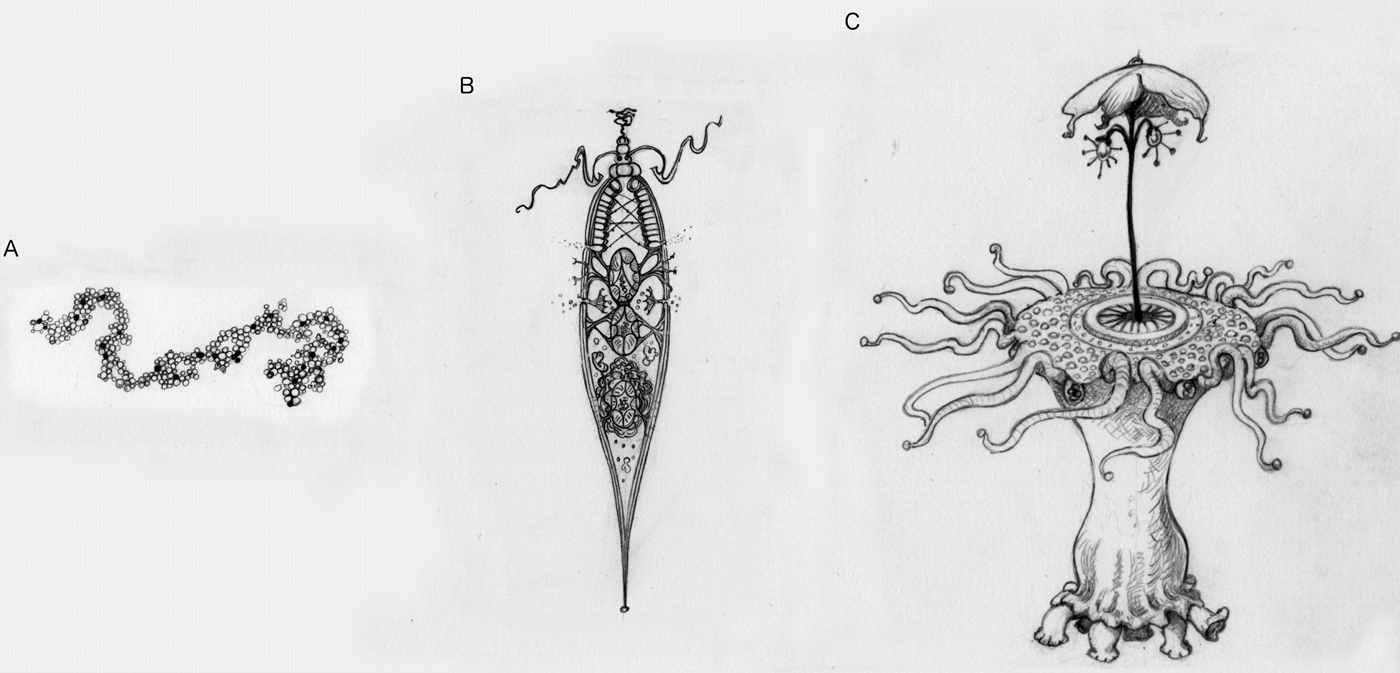
Fig. 2. Picture an alien. These illustrations represent different levels of adaptive complexity we might imagine when thinking about aliens. (a) A simple replicating molecule, with no apparent design. This may or may not undergo natural selection. (b) An incredibly simple, cell-like entity. Even something this simple has sufficient contrivance of parts that it must undergo natural selection. (c) An alien with many intricate parts working together is likely to have undergone major transitions.
Complexity
What is complexity?
We have established that aliens will undergo natural selection. It also seems reasonable that, given the sliding scale from replicating molecules to large creatures with many ‘body parts’, and beyond, some alien discoveries would be more interesting than others. In particular, the more complex the aliens we find, the more interesting and exciting they will be, irrespective of whether they appear anything like the life forms on the Earth. Something similar to a colony of Ewoks from Star Wars or the Octomite in Fig. 4 would likely be more interesting than a simple chemical replicator.
Complexity is difficult to define, and there is certainly no hard and fast rule about what is and is not complex. In biology, it is common to define complexity in terms of functional parts. Things with more parts taking on more tasks and containing more functional interactions are more complex (Maynard Smith & Szathmary Reference Smith and Szathmáry1995; Corning & Szathmáry Reference Corning and Szathmáry2015). A tree is more complex than a virus, and a beehive is more complex than a protein. Importantly, with organisms as with machines, the parts need to be working towards a common purpose, such as assembling a car or surviving to reproduce. Again, our goal here is not to provide definitions. The challenge comes at the boundaries, for example between a virus and a cell, where the definitions become murky. In the following sections, we are not focusing on the boundaries, but things, like the vast majority of life on the Earth, which clearly have a multitude of parts working in concert. Astrobiology is a largely empirical field, and the kinds of things programs like SETI are searching for are undeniably complex.
Complexity on Earth
What do we know about how complexity arises on the Earth? The theory of natural selection itself is silent about whether complexity will arise. The theory is useful for making predictions about what kinds of conditions or environments will lead to what kinds of evolutionary adaptations – not for making long-term predictions about the form of specific traits or creatures. However, recent advances in the field of evolutionary biology have shed light on how complexity has arisen on the Earth, on what points on the tree of life this has happened, and on what theoretical conditions favour it (Maynard Smith & Szathmáry Reference Smith and Szathmáry1995; Queller Reference Queller1997; Bourke Reference Bourke2011; West et al. Reference West, Fisher, Gardner and Kiers2015).
In particular, the evolution of complex life on the Earth appears to have depended upon a small number of what have been termed major evolutionary transitions in individuality. In each transition, a group of individuals that could previously replicate independently cooperate to form a new, more complex life form or higher level organism. For example, genes cooperated to form genomes, different single-celled organisms formed the eukaryotic cell, cells cooperated to form multicellular organisms, and multicellular organisms formed eusocial societies (Maynard Smith & Szathmáry Reference Smith and Szathmáry1995; Queller Reference Queller1997; Bourke Reference Bourke2011; West et al. Reference West, Fisher, Gardner and Kiers2015).
Major transitions
Major transitions on the Earth
Major evolutionary transitions are defined by two features. First, entities that were capable of replication before the transition can replicate only as part of a larger unit after it (interdependence). For example, the cells in our bodies cannot evolve back into single-celled organisms. Second, there is a relative lack of conflict within the larger unit, such that it can be thought of as an organism (individual) in its own right (Queller & Strassmann Reference Queller and Strassmann2009; West et al. Reference West, Fisher, Gardner and Kiers2015). For example, it is common to think of a single bird as an individual, and not as a huge community of cells each doing their own thing.
Major transitions are important because the new higher-level organisms that they produce can lead to a great jump in complexity. For example, the evolution of multicellularity involved a transition from an entity with one part (the single-celled organism) working for the success of itself, to an entity with many parts (the multicellular organism), working for the success of the whole group. The cells can now have very different functions (a division of labour), as each is just a component of a multicellular machine, sacrificing itself for the good of the group, to get a sperm or egg cell into the next generation. As a result, diverse specialized forms such as eyes, kidneys, and brains were able to develop. The rise in complexity on Earth has been mediated by a handful of such jumps, when units with different goals (genes, single cells, individual insects) became intricately linked collectives with a single common goal (genomes, multicellular organisms, eusocial societies). Increases in complexity can also occur through mutations, gene duplications, or even whole genome duplications, but these are not major transitions. These other changes tend to be reversible and gradual, while major transitions are irreversible and cause large leaps in complexity.
The identification of major evolutionary transitions was an empirical observation about how complexity has increased on earth (Maynard Smith & Szathmáry Reference Smith and Szathmáry1995). The next step was to use evolutionary theory to provide insight about when (or under what conditions) we can expect major transitions to occur (Maynard Smith & Szathmáry Reference Smith and Szathmáry1995; Queller Reference Queller1997; Gardner & Grafen Reference Gardner and Grafen2009; Bourke Reference Bourke2011; West et al. Reference West, Fisher, Gardner and Kiers2015). Major transitions involve the original entities completely subjugating their own interests for the interests of the new collective. This represents an incredibly extreme form of cooperation. Think of the skin or liver cells in your body sacrificing for your sperm or eggs, or the worker ants in a eusocial colony sacrificing for the queen. Evolutionary theory tells us what conditions lead to such extraordinary cooperation.
What conditions drive major transitions?
Consider a multicellular organism, such as yourself. Why don't your hand and heart cells try to reproduce themselves, as opposed to helping your sperm or egg cells? The answer involves genetic similarity or ‘relatedness’ (Hamilton Reference Hamilton1964). Your hand cells contain the same genes as your sperm cells because they are clonal copies. A hand cell could in principle get the same fraction of its genes into the next generation (all of them) by either copying itself, or by helping copy the sperm cells. A similar phenomenon occurs in eusocial insects, such as some ants, bees, wasps and termites. A worker termite can pass on half her genes to her offspring. But a random sibling in the colony (her brother or sister) also contains, on average, half her genes. Thus, a worker can get the same fraction of gene copies into the next generation by reproducing or by helping her mother, the queen, to reproduce (Hamilton Reference Hamilton1964; Boomsma Reference Boomsma2009). Helping their mother is likely to be more efficient than reproducing on their own, and so our termite can better get their genes into the next generation by helping rather than reproducing (Hamilton Reference Hamilton1964; Queller & Strassmann Reference Queller and Strassmann1998; Bourke Reference Bourke2011).
These are two examples of alignment of interests. The ‘interests’ are evolutionary interests in getting genes into future generations. The hand and the sperm cells both act as if they ‘want’ to get copies of their genes into the next generation, because as we discussed above, natural selection will have led to them being adapted in this way (Grafen Reference Grafen2003; Gardner Reference Gardner2009). The interests between them are aligned because they share the same genes. When individuals share genes, we say that they are genetically related. Relatedness is a statistical measure of the extent to which individuals share genes (Grafen Reference Grafen1985).
In the case of eusocial ant colonies and human bodies, the interests are aligned through genetic relatedness. But there are other ways for evolutionary interests to be aligned. Consider, for example, a mutualism between two species. Some aphids carry bacteria in their gut (Moran Reference Moran2007). The aphids provide the bacteria with sugars and other nutrients to survive and the bacteria provide the aphids with vital amino acids missing from their diet. The aphid and the bacteria do not share the same genes, but neither can reproduce without the other. To reproduce itself, the aphid has to help reproduce the bacteria and vice versa. Again, their evolutionary interests are aligned.
The very cells that make up our bodies – known as eukaryotic cells – evolved through a similar kind of alignment of interests (Margulis Reference Margulis1970; Thiergart et al. Reference Thiergart, Landan, Schenk, Dagan and Martin2012; Archibald Reference Archibald2015). Early in the evolution of life, one bacterial species engulfed another. Over time, the two species took on different roles, with one specializing in replication and the other in energy production. The nucleus of our cells is the descendant of the former, and the mitochondria the latter. Neither can reproduce without the other. Their interests are aligned through reproductive dependence on each other.
All cooperation in nature requires alignment of interests (West et al. Reference West, Griffin and Gardner2007). Consider, for example, flower pollination by bees. The bee benefits by receiving food from the flower, and the flower benefits by being pollinated. But major transitions are a particularly extreme form of cooperation. Compare the pollination scenario to the cells within the flower or the bee. Major transitions involve organisms cooperating so completely that they give up their status as individuals, becoming parts of a whole (Queller & Strassmann Reference Queller and Strassmann2009). Unsurprisingly, then, major transitions require the extreme condition of effectively complete or perfect alignment of interests (Gardner & Grafen Reference Gardner and Grafen2009; West et al. Reference West, Fisher, Gardner and Kiers2015).
It is also useful to consider the biology of organisms that do not have interests sufficiently aligned, and thus where conflict remains and major transitions have not occurred. For example, in single-celled organisms, we can compare non-clonal cooperative groups of things like slime moulds with clonal groups such as those that make up multicellular organisms such as humans and trees. These non-clonal groups have evolved only relatively limited division of labour, and never complex multicellular organisms (Fisher et al. Reference Fisher, Cornwallis and West2013). Numerous experimental studies have shown that this is because in non-clonal groups non-cooperative ‘cheats’ can spread, limiting the extent of cooperation (Griffin et al. Reference Griffin, West and Buckling2004; Diggle et al. Reference Diggle, Griffin, Campbell and West2007; Kuzdzal-Fick et al. Reference Kuzdzal-Fick, Fox, Strassmann and Queller2011; Rumbaugh et al. Reference Rumbaugh, Trivedi, Watters, Burton-Chellew, Diggle and West2012; Pollitt et al. Reference Pollitt, West, Crusz, Burton-Chellew and Diggle2014; Popat et al. Reference Popat, Pollitt, Harrison, Naghra, Hong, Chan, Griffin, Williams, Brown, West and Diggle2015; Inglis et al. Reference Inglis, Ryu, Asikhia, Strassmann and Queller2017).
Thus, there must be something in place to maintain the alignment of interests (Bourke Reference Bourke2011; West et al. Reference West, Fisher, Gardner and Kiers2015). Evolutionary theory can suggest what these somethings would have to be. In multicellular organisms, the something is the single-celled bottleneck (Buss Reference Buss1987; Queller Reference Queller2000). Multicellular organisms start each new generation as a single-celled zygote, such that all the cells in the resulting body are clonal (it could also be a spore giving rise to a haploid cell). Eusocial insect colonies evolved from colonies founded by a singly mated queen (Boomsma Reference Boomsma2007, Reference Boomsma2009, Reference Boomsma2013; Hughes et al. Reference Hughes, Oldroyd, Beekman and Ratnieks2008). If the queen had multiple mating partners, a worker would have half-sisters, and be less related to her siblings than her offspring, breaking down the alignment. The monogamous mating pair is the eusocial colony's equivalent of a zygote or a bottlenecking event (Boomsma Reference Boomsma2013). With unrelated units, like mitochondria and the nucleus, the individual parts must be co-dependent for joint reproduction (Foster & Wenseleers Reference Foster and Wenseleers2006; West et al. Reference West, Fisher, Gardner and Kiers2015) – which can be thought of as a different form of bottleneck. The rarity of conditions like these – conditions under which alignment is so complete – explains the rarity of major transitions in individuality in the history of life.
Biology of organisms that have undergone major transitions
Do the conditions required for major transitions tell us anything about the biology of organisms that have undergone major transitions? Yes. Organisms are a nested hierarchy, where each nested level is the vestige of a former individual (Fig. 3). Eusocial ant colonies function as a single individual, but are made up of multicellular organisms. Those organisms themselves are made up of cells. In turn, those cells resulted from the fusion of two simple species early in evolution. Each of those organisms had a genome that evolved from the union of the individual, replicating molecules.
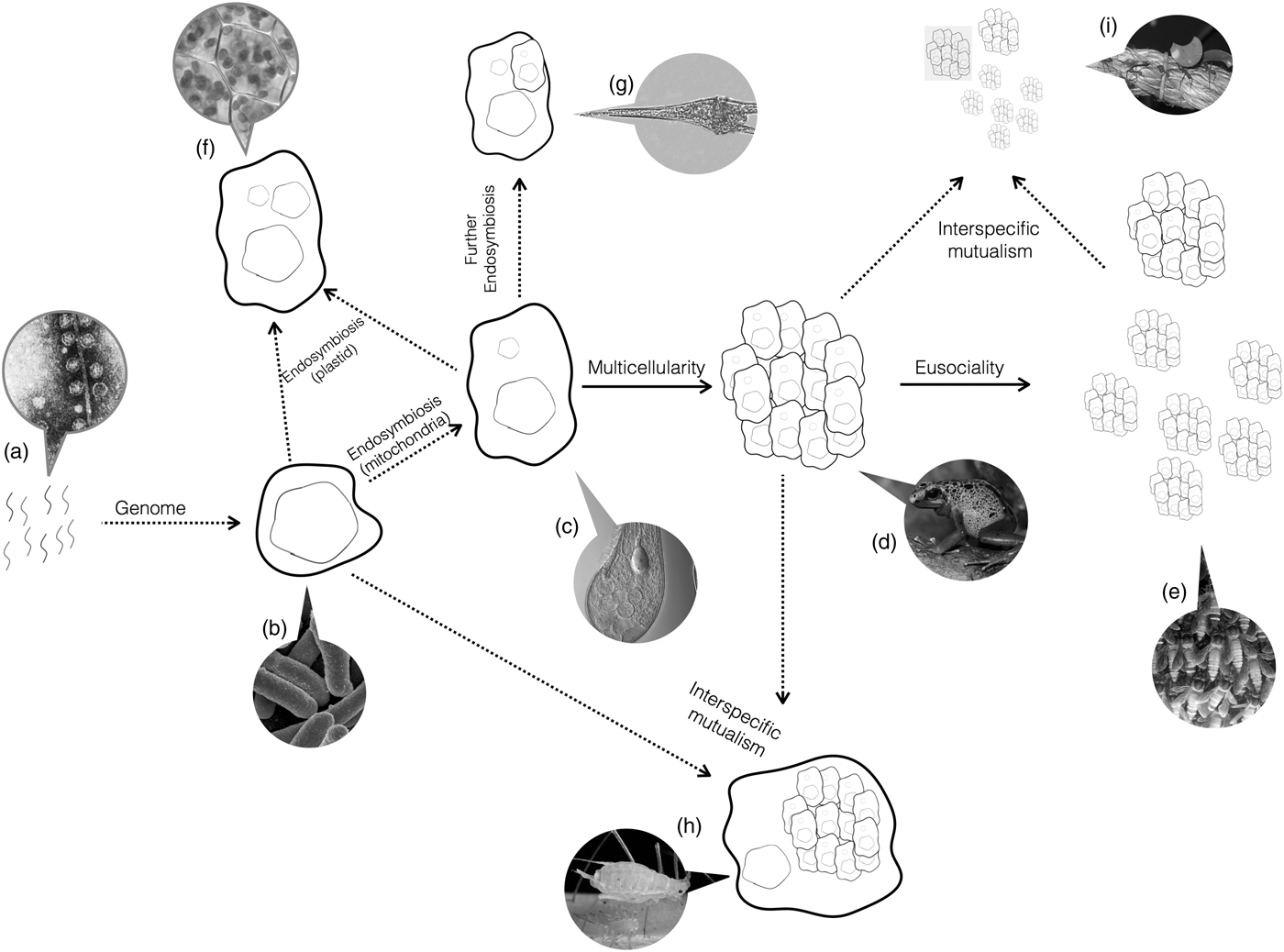
Fig. 3. Major Transitions. Life started with naked replicating molecules, and has since undergone a series of major transitions. Arrows show the occurrence of major transitions in individuality. Dotted arrows represent transitions between dislike things and solid lines represent transitions between like things. Callouts show examples of the present-day organisms that have undergone that transition but no further ones. (a) As we have not yet identified the earliest replicators, Spiegelman's monster, a simple replicating RNA molecule, is shown as an example candidate. (b) A single-celled bacteria, such as Escherichia coli. (c) A single-celled eukaryote, like Blepharisma japonicum. (d) A multicellular organism, like frogs. (e) An obligate eusocial colony, such as honeybees. (f) Secondary endosymbiosis events, such as the origin of the chloroplast. (g) Further endosymbiosis events, such as those leading to Dinoflagellates. (h) Obligate interspecific mutualisms, such as between aphids and buchnera bacteria. (i) Obligate mutualisms between a multicellular organism and eusocial colony, such as between leaf-cutter ants and fungi. All images courtesy of Wikipedia.
Further, at each level of the hierarchy, there must be something to align the interests of the parts. This usually happens through some form of population bottlenecking. When the parts are related, it is a relatedness bottleneck, such as the single-celled stage in multicellular organisms, or the singly mated female in the social insects (Boomsma Reference Boomsma2009, Reference Boomsma2013; West et al. Reference West, Fisher, Gardner and Kiers2015). When the parts are unrelated, it is usually another form of a bottleneck, such as enforced vertical transmission with joint reproduction (Foster & Wenseleers Reference Foster and Wenseleers2006; West et al. Reference West, Fisher, Gardner and Kiers2015). We use the term ‘bottleneck’ to refer to new generations being founded by a strict unit (the zygote, the mutualist pair, etc.), but another way to think of this is that the parts require each other for reproduction (e.g. the soma and the germ line, or the mitochondria and the nucleus). Other, further aligners may be required (e.g. in multicellular organisms, there may need to be a cap on somatic mutations), but these are more likely to be life-form specific.
To conclude so far, empirical observation tells us that complexity has increased on earth through major transitions. Evolutionary theory tells us that for major transitions to occur, the conflict must be eliminated. The theory also tells us what conditions lead to the elimination of conflict. The empirical data agree with the predictions of the theory, in that major transitions have only occurred in the extreme conditions that effectively remove conflict (Boomsma Reference Boomsma2007; Hughes et al. Reference Hughes, Oldroyd, Beekman and Ratnieks2008; Fisher et al. Reference Fisher, Cornwallis and West2013; West et al. Reference West, Fisher, Gardner and Kiers2015; Fisher et al. Reference Fisher, Henry, Cornwallis, Kiers and West2017).
Complex aliens
Complexity and major transitions in space
We can now ask: what does the major evolutionary transition approach tell us about aliens? Will extraterrestrial life undergo major transitions? Not necessarily. Natural selection cannot predict a specific course of evolution. However, as we have said, we might be particularly interested in complex aliens. Complexity requires different parts or units working together towards a common goal or purpose. Under natural selection, units are selected to be selfish, striving to replicate themselves at the expense of others. Theory tells us that for units to unite under a common purpose, the evolutionary conflict between them must effectively eliminate (Gardner & Grafen Reference Gardner and Grafen2009; West et al. Reference West, Fisher, Gardner and Kiers2015).
Once again, picture an alien (Fig. 2). If you are picturing something like unlinked replicating molecules or undifferentiated blobs of slime, then your aliens might not have undergone major transitions. But if what you are picturing has different parts with specialized functions, then your alien is likely to have undergone major transitions (Fig. 2c). What matters is not that we call them ‘major transitions’, but rather that complexity requires multiple parts of an organism striving to the same purpose, and that theory predicts that this requires restrictive conditions (Gardner & Grafen Reference Gardner and Grafen2009; West et al. Reference West, Fisher, Gardner and Kiers2015). Consequently, if we find complex organisms, we can make predictions about what they will be like.
Are there other ways to get complexity? To do so, natural selection would have to sculpt separate parts with unique functions out of a single replicator. Could, for example, the alien equivalent of a single copy of a gene, housed in one ‘cell’ generate the equivalent of limbs and organs? If so, it would disprove our prediction. However, both empirical (major transitions are how complexity has increased on Earth) and theoretical (functional parts requires the elimination of conflict) evidence support the argument that complex aliens will have undergone major transitions.
The biology of complex aliens
Given that complex aliens will have undergone major transitions, we can make a number of predictions about their biology (Fig. 4).
1. They will be entities that are made up of smaller entities – a nested hierarchy of individuality with as many levels as completed transitions. This could mean a collection of replicators, like the first genomes on the Earth, or some hideously complex nesting of groups on a planet where many more transitions have occurred than on our own. For example, you might imagine a ‘society of societies’, where many different social colonies collaborate, with each society specializing on different tasks, such that they are completely dependent on each other. Versions of the simpler entities are likely to be found free-living on the planet as well.
2. Whatever the number of transitions, there will be something that aligns interests, or eliminates conflict within the entities, at the level of each transition.
3. Theory suggests that some sort of population bottlenecking will be key to aligning interests. Bottlenecking is not necessarily the only way to eliminate conflict, but it is probably the easiest evolutionary route to take. In particular, it does not require additional mechanisms of enforcement, such as kin discrimination, policing or randomization. The specific kinds of bottlenecking will depend on whether like or dislike units are united.
a. When like entities come together, interests can be aligned through a bottleneck similar to our single-celled bottleneck in multicellular organisms or the single mating pair in eusocial colonies, which maximizes relatedness between entities.
b. If the organisms are made up different types of entities, we can expect something similar to the bottleneck that forces mitochondria and nuclei to pass to the next generation together, with joint reproduction. By trapping individuals together over evolutionary time, their interests become aligned.
c. Some aliens, like us, may contain both types of conflict reduction, for having both like and dislike types joined within them.
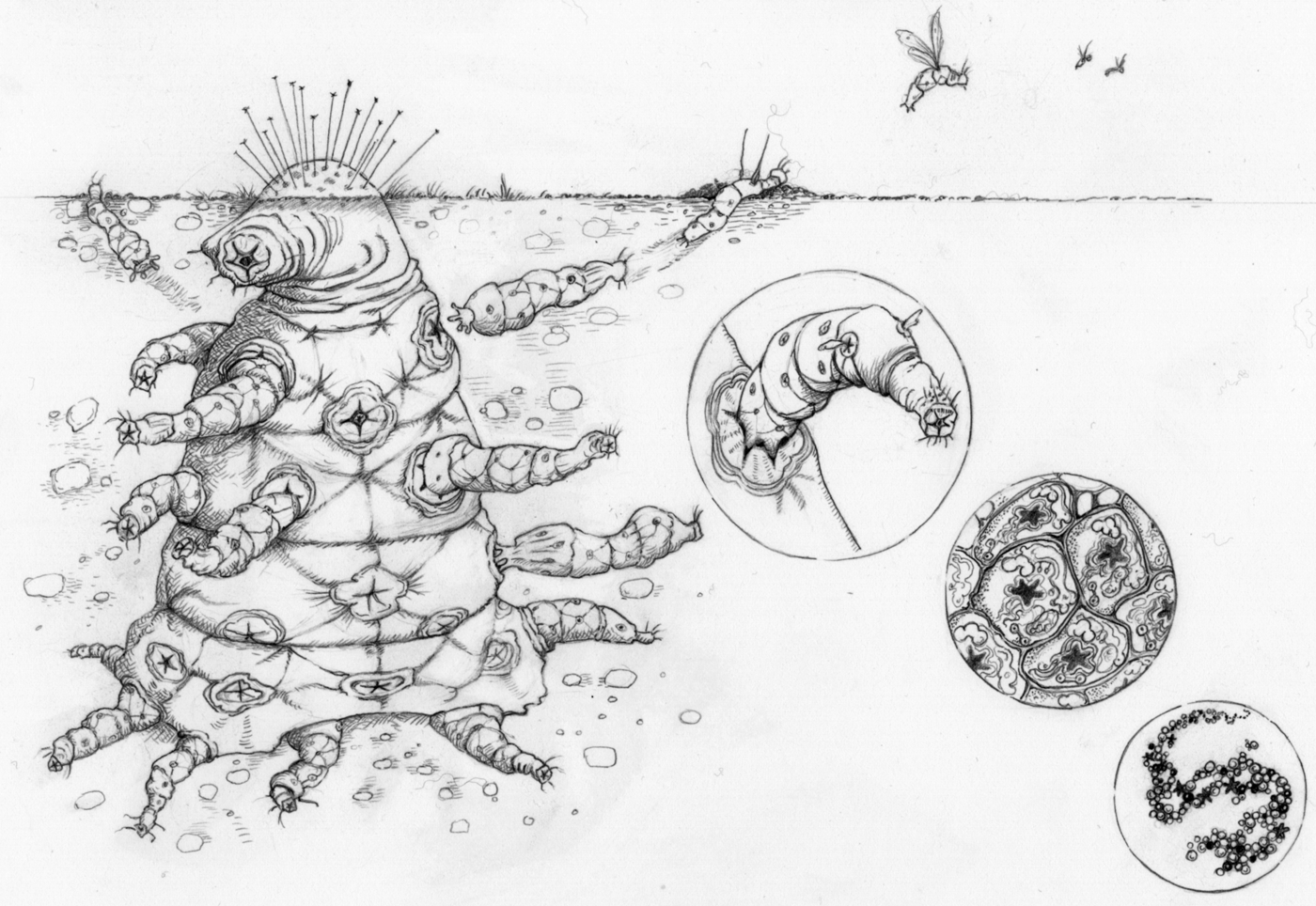
Fig. 4. Major transitions in space: ‘The Octomite’. A complex alien that comprises a hierarchy of entities, where each lower-level collection of entities has aligned evolutionary interests such that conflict is effectively eliminated. These entities engage in a division of labour, with various parts specializing on various tasks, such that the parts are mutually dependent.
Conclusion
When using evolutionary theory to make predictions about extraterrestrial life, it is important to avoid circularity. Our chain of argument is: (1) Extraterrestrial life will have undergone natural selection. (2) Knowing that aliens undergo natural selection, we can make further predictions about their biology, based on the theory of natural selection. In particular, we can say something about complex aliens – that they will likely have undergone major transitions. (3) Theory tells us that restrictive conditions, which eliminate conflict, are required for major transitions. (4) Consequently, complex aliens will be composed of a nested hierarchy of entities, with the conditions required to eliminate conflict at each of those levels.
When making predictions about aliens, we must take advantage of our entire scientific toolkit. Mechanistic understanding is a good way to extrapolate from what we see on Earth. The theory is a good way to make predictions that are independent of the details of the Earth. Combining both approaches is the best way to make predictions about the many hundreds, thousands or millions of hypothetical aliens. Now we just need to find them.
Acknowledgements
We thank The Clarendon Fund, Hertford College, and the Natural Environment Research Council for funding; and Magdalen College for emergency housing.
Author disclosure statement
No competing financial interests exist.






