Published online by Cambridge University Press: 09 February 2012
When droplets of dilute suspensions are left to evaporate the final dry residue is typically the familiar coffee-ring stain, with nearly all material deposited at the initial triple line (Deegan et al., Nature, vol. 389, 1997, pp. 827–829). However, aqueous poly(ethylene oxide) (PEO) droplets only form coffee-ring stains for a very narrow range of the experimental parameters molecular weight, concentration and drying rate. Instead, over a wide range of values they form either a flat disk or a very distinctive tall central monolith via a four-stage deposition process which includes a remarkable bootstrap-building step. To predict which deposit will form, we present a quantitative model comparing the effects of advective build-up at the triple line to diffusive flux and use this to calculate a dimensionless number 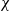 . By experimentally varying concentration and flux (using a low-pressure drying chamber), the prediction is tested over nearly two orders of magnitude in both variables and shown to be in good agreement with the boundary between disks and monoliths at
. By experimentally varying concentration and flux (using a low-pressure drying chamber), the prediction is tested over nearly two orders of magnitude in both variables and shown to be in good agreement with the boundary between disks and monoliths at 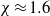 .
.
Low pressure drying of 1.5% polymer solution droplet. A 10 μl droplet of PEO in water with initial concentration c0=0.015 is shown drying at a reduced pressure of 25mbar. The triple line of the droplet begins to recede at around 80 seconds, marking the start of stage 2. The remaining liquid begins to lift at 240 seconds (start of stage 3) and is completely enclosed by a dry skin at 400 seconds (start of stage 4).
Low pressure drying of 1.5% polymer solution droplet. A 10 μl droplet of PEO in water with initial concentration c0=0.015 is shown drying at a reduced pressure of 25mbar. The triple line of the droplet begins to recede at around 80 seconds, marking the start of stage 2. The remaining liquid begins to lift at 240 seconds (start of stage 3) and is completely enclosed by a dry skin at 400 seconds (start of stage 4).
Low pressure drying of 5% polymer solution droplet. A 10 μl droplet of PEO in water with initial concentration c0=0.05 is shown drying at a reduced pressure of 25mbar. The triple line of the droplet begins to recede at around 10 seconds (start of stage 2), then the remaining liquid begins to lift at 120 seconds (start of stage 3) and is completely enclosed by a dry skin at 400 seconds (start of stage 4). Stages 2 and 3 start noticeably earlier for this droplet than for the lower concentration droplet with c0=0.015.
Low pressure drying of 5% polymer solution droplet. A 10 μl droplet of PEO in water with initial concentration c0=0.05 is shown drying at a reduced pressure of 25mbar. The triple line of the droplet begins to recede at around 10 seconds (start of stage 2), then the remaining liquid begins to lift at 120 seconds (start of stage 3) and is completely enclosed by a dry skin at 400 seconds (start of stage 4). Stages 2 and 3 start noticeably earlier for this droplet than for the lower concentration droplet with c0=0.015.