Published online by Cambridge University Press: 14 June 2019
We present a numerical analysis of the rheology of a suspension of red blood cells (RBCs) in a wall-bounded shear flow. The flow is assumed as almost inertialess. The suspension of RBCs, modelled as biconcave capsules whose membrane follows the Skalak constitutive law, is simulated for a wide range of viscosity ratios between the cytoplasm and plasma, 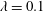 $\unicode[STIX]{x1D706}=0.1$–10, for volume fractions up to
$\unicode[STIX]{x1D706}=0.1$–10, for volume fractions up to 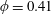 $\unicode[STIX]{x1D719}=0.41$ and for different capillary numbers (
$\unicode[STIX]{x1D719}=0.41$ and for different capillary numbers (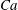 $Ca$). Our numerical results show that an RBC at low
$Ca$). Our numerical results show that an RBC at low 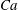 $Ca$ tends to orient to the shear plane and exhibits so-called rolling motion, a stable mode with higher intrinsic viscosity than the so-called tumbling motion. As
$Ca$ tends to orient to the shear plane and exhibits so-called rolling motion, a stable mode with higher intrinsic viscosity than the so-called tumbling motion. As 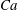 $Ca$ increases, the mode shifts from the rolling to the swinging motion. Hydrodynamic interactions (higher volume fraction) also allow RBCs to exhibit tumbling or swinging motions resulting in a drop of the intrinsic viscosity for dilute and semi-dilute suspensions. Because of this mode change, conventional ways of modelling the relative viscosity as a polynomial function of
$Ca$ increases, the mode shifts from the rolling to the swinging motion. Hydrodynamic interactions (higher volume fraction) also allow RBCs to exhibit tumbling or swinging motions resulting in a drop of the intrinsic viscosity for dilute and semi-dilute suspensions. Because of this mode change, conventional ways of modelling the relative viscosity as a polynomial function of 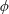 $\unicode[STIX]{x1D719}$ cannot be simply applied in suspensions of RBCs at low volume fractions. The relative viscosity for high volume fractions, however, can be well described as a function of an effective volume fraction, defined by the volume of spheres of radius equal to the semi-middle axis of a deformed RBC. We find that the relative viscosity successfully collapses on a single nonlinear curve independently of
$\unicode[STIX]{x1D719}$ cannot be simply applied in suspensions of RBCs at low volume fractions. The relative viscosity for high volume fractions, however, can be well described as a function of an effective volume fraction, defined by the volume of spheres of radius equal to the semi-middle axis of a deformed RBC. We find that the relative viscosity successfully collapses on a single nonlinear curve independently of 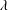 $\unicode[STIX]{x1D706}$ except for the case with
$\unicode[STIX]{x1D706}$ except for the case with 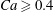 $Ca\geqslant 0.4$, where the fit works only in the case of low/moderate volume fraction, and fails in the case of a fully dense suspension.
$Ca\geqslant 0.4$, where the fit works only in the case of low/moderate volume fraction, and fails in the case of a fully dense suspension.
Tumbling RBC for Ca = 0.05 and λ = 5.0. The initial orientation angle is Ψ0 = π/2.
Rolling RBC for Ca = 0.05 and λ = 5.0. The initial orientation angle is Ψ0 = rand. (at least Ψ0 ≠ 0 or ≠ π/2).
RBC with complex deformed shape for Ca = 1.2 and λ = 5.0. The initial orientation angle is Ψ0 = π/4.
RBC with complex deformed shape for Ca = 0.8 and λ = 5.0. The initial orientation angle is Ψ0 = π/4.
RBC with periodic motion (kayaking motion) for Ca = 0.2 and λ = 0.1. The initial orientation angle is Ψ0 = π/4.
Semi-dilute suspension (φ = 0.05) of RBCs for Ca = 0.05 and λ = 5.0.
Dense suspension (φ = 0.41) of RBCs for Ca = 0.05 and λ = 5.0.