Article contents
Competitive interplay of deposition and etching processes in atomic layer growth of cobalt and nickel metal films
Published online by Cambridge University Press: 05 November 2018
Abstract
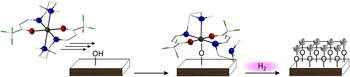
Atomic layer deposition (ALD) of air stable cobalt and nickel complexes based on tridentate enaminones N,N-(4,4,4-trifluorobut-1-en-3-on)-dimethylethyldiamine (Htfb-dmeda) and N,N-(4,4,4-trifluorobut-1-en-3-on)-dimethylpropyldiamine (Htfb-dmpda) successfully produced metallic cobalt and nickel thin films. Detailed X-ray photoelectron spectroscopy (XPS) studies on the binding interaction of the first precursor monolayer with the surface functional groups elucidated the chemisorption behavior of the new precursor systems. A reactive remote hydrogen plasma was used as the co-reactant to activate the precursor decomposition yielding metal hydroxide intermediates. Subsequent hydrogen plasma etching of as-deposited films resulted in phase-pure metallic films through a recrystallization process, verified by surface and sub-surface XPS. Scanning electron microscopy (SEM) and atomic force microscopy (AFM) analyses revealed pinhole-free films, with low surface roughness (0.2 ± 0.06 nm root mean square, RMS) for both, cobalt and nickel thin films. Herein, the competitive role of hydrogen as etchant and reactant was demonstrated as prolonged plasma exposure time periods resulted in the formation of metal hydrides. This is mostly due to the catalytic dissociation of molecular hydrogen on transition metal surfaces, which already occurs upon low energy input.
Information
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2018
Footnotes
These authors contributed equally to this work.
References
REFERENCES
- 6
- Cited by

