Published online by Cambridge University Press: 02 January 2018
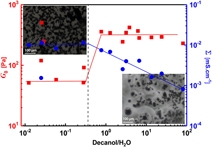
In an attempt to introduce a novel approach to formulate carbon black (ketjen black) suspension with enhanced colloidal stability, improved flowability, and higher conductivity, ketjen black was dispersed in microemulsion systems composed of a non-ionic surfactant (Triton X100), decanol, and water. Rheo-electric and rheo-microscopy proved to be very powerful techniques that are able to elucidate the microstructure evolution with the composition and under shear flow. Interestingly, the carbon black slurries at low decanol/water ratio are weak gels (flowable) with higher electrical conductivity than those at higher ratio, which shows strong-gel viscoelastic response. In addition, the slurries show recoverable electrical behavior under shear flow in tandem with the viscosity trend. It is likely that the oil-in-water microemulsion enhances slurries’ stability without affecting the percolating network of carbon black. On the other hand, the oil-in-water analogous and bilayer structure of the lamellar phase makes the slurries less conductive as a consequence of losing the network percolation.
Contributing Editor: Yat Li
Please note a has been issued for this article.