Article contents
Development of multilayered biomimetic bone plates: In vitro release assessment
Published online by Cambridge University Press: 21 January 2019
Abstract
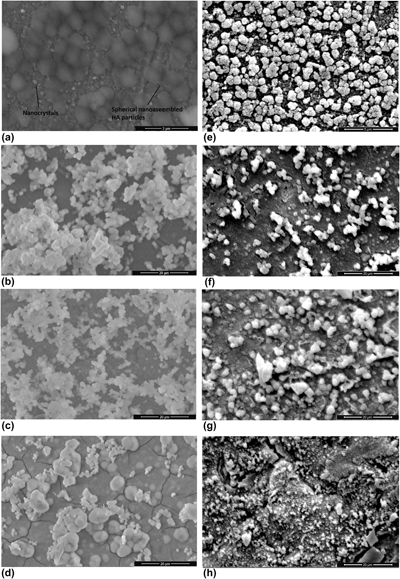
In this study, Sr-incorporated nano-assembled hydroxyapatite structures (HASr) on 316L stainless steel bone plates were prepared by a biomimetic method induced by 10× simulated body fluid (SBF). First, HASr was coated on bone plates by the interaction of ions with 10× SBF containing different concentration of strontium ions. Then, silver coating is achieved as a second layer on bone plates. The cumulative release of strontium ions (Sr2+) and silver ions (Ag+) from multilayered HASr-Ag bone plates at the end of 15 days was in the range of 0.016–0.085 mM and 0.064–0.135 mM, respectively. The release mechanism for the bone plates was evaluated by several mathematical models that best fit the release data. The results showed that Sr2+ and Ag+ are released from multilayered bone plates by diffusion, whereas the release of Ag+ is not occurred by diffusion, instead the mechanism is dissolution, when silver is coated alone on bone plates.
Information
- Type
- Article
- Information
- Journal of Materials Research , Volume 34 , Issue 11: Focus Issue: (Nano)materials for Biomedical Applications , 14 June 2019 , pp. 1879 - 1891
- Copyright
- Copyright © Materials Research Society 2019
References
- 2
- Cited by

