Article contents
Standard Gibbs energy of formation of Pb2Ru2O6.5
Published online by Cambridge University Press: 03 March 2011
Abstract
Lead ruthenate is used as a bifunctional electrocatalyst for both oxygen evolution and reduction and as a conducting component in thick-film resistors. It also has potential applications in supercapacitors and solid oxide fuel cells. However, thermodynamic properties of the compound have not been reported in the literature. The standard Gibbs energy of formation has now been determined in the temperature range from 873 to 1123 K using a solid-state cell incorporating yttria-stabilized zirconia (YSZ) as the electrolyte, a mixture of PbO + Pb2Ru2O6.5 + Ru as the measuring electrode, and Ru + RuO2 as the reference. The design of the measuring electrode is based on a study of phase relations in the ternary system Pb–Ru–O at 1123 K. For the reaction,
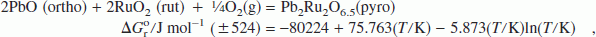
the standard enthalpy of formation and standard entropy at 298.15 K are estimated from the high-temperature measurements. An oxygen potential diagram for the system Pb–Ru–O is composed based on data obtained in this study and auxiliary information from the literature
Keywords
Information
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2007
References
REFERENCES
- 3
- Cited by

