Article contents
Effect of sterilization processes on the properties of a silane hybrid coating applied to Ti6Al4V alloy
Published online by Cambridge University Press: 21 November 2017
Abstract
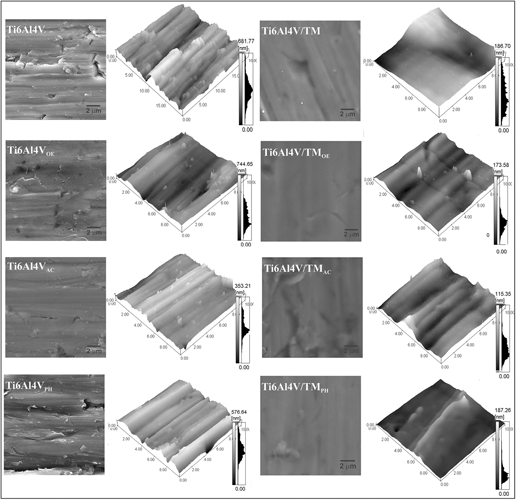
Sterilization is one of the last stages prior to the implantation of a biomaterial. Therefore, the method should be chosen carefully as this is determinant not to compromise the properties of the material. In this context, three sterilization processes were evaluated as to their effect on the properties of a silane hybrid coating: steam autoclave, ethylene oxide, and hydrogen peroxide plasma. The coating was obtained from a sol consisting of alkoxysilane Tetraethoxysilane and organoalcoxysilane Methyltriethoxysilane (MTES), applied to the Ti6Al4V substrate, to increase its corrosion resistance and biocompatibility. After sterilization, the samples were characterized by scanning electron microscopy, atomic force microscopy, profilometry, wetabillity, and Fourier transform infrared spectroscopy. The electrochemical behavior was monitored by open circuit potential and potentiodynamic polarization curves. The cytocompatibility was evaluated by adhesion, viability, and morphological alterations in the MG-63 cells. The results showed that the protective behavior of the hybrid coating was compromised regardless of the sterilization method. However, the steam autoclave caused more morphological changes on the silane hybrid coating as well as on the Ti6Al4V substrate than the other two sterilization methods. Although the sterilized hybrid coating did not show cytotoxicity, the hybrid coating sterilized by hydrogen peroxide plasma showed a higher percentage of viable cells. The ethylene oxide presented the lowest percentage of viability and the highest cell death rate.
Keywords
Information
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2017
Footnotes
Contributing Editor: Amit Bandyopadhyay
References
REFERENCES
- 10
- Cited by


