Published online by Cambridge University Press: 15 February 2016
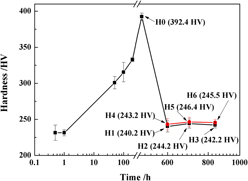
The evolution behavior of Ni2(Cr, Mo) phase with Pt2Mo-type structure in the Ni–Cr–Mo alloy with a low atomic Mo/Cr ratio subjected to a long-term thermal exposure of 100–340 h at 600 °C was investigated using transmission electron microscopy and microhardness. Results demonstrate that there is a linear relationship between major axis cube (L 3) of ordered domain and thermal exposure time (t) followed by a coarsening regime described by the Lifshitz–Slyozov–Wagner model, as well as between the aspect ratio (D) of ordered domain and thermal exposure time. The volume fraction of ordered domain increases with increasing thermal exposure time, whereas the hardening of samples decreases due to growth-coarsening of ordered domain. Prolonged thermal exposure time led to the coarsening of ordered domain by rate of (3.39 ± 0.02) × 10−30 m3/s without changing their crystallography and ordering characteristics during thermal exposure. Plastic deformation before thermal exposure do not lead to decomposition of initial Ni2(Cr, Mo) phase, but both plastic deformation and thermal exposure affect their morphology and distribution.