Article contents
Exploration of interactions of ‘blood-nano interface’ of carbon-based nanomaterials for biomedical applications
Published online by Cambridge University Press: 31 May 2019
Abstract
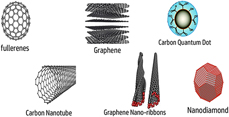
One of the most promising nanoscale materials which fascinated researchers for the last few decades owing to its unique optoelectronics and physicochemical properties are carbon-based nanomaterials (CBNs). Various forms of CBNs have been developed such as single and multi-walled carbon nanotubes, graphene, fullerenes, nanodiamonds, and fluorescent carbon quantum dots (C-Dots) whereas each form is having its own exceptional properties owing to its dimensionalities and architectures. The advent of these unique classes of nanoscale materials opens up a spectrum of new opportunities and possibilities in employing these in emerging areas of biomedical. However, successful biomedical applications greatly rely on the likelihood of the comprehensive understanding of physicochemical interactions and biological responses of CBNs. Herein, we have tried to explore the ‘blood-CBNs’ interface by including the findings of recent studies. The role of surface modifications and functionalization in order to mitigate the adverse outcomes has also been incorporated.
Keywords
Information
- Type
- REVIEW
- Information
- Journal of Materials Research , Volume 34 , Issue 11: Focus Issue: (Nano)materials for Biomedical Applications , 14 June 2019 , pp. 1950 - 1964
- Copyright
- Copyright © Materials Research Society 2019
Footnotes
These authors contributed equally to this work.
This section of Journal of Materials Research is reserved for papers that are reviews of literature in a given area.
References
- 2
- Cited by

