Published online by Cambridge University Press: 27 September 2019
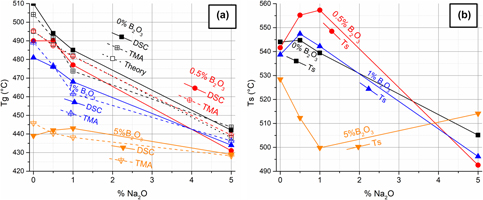
In the recent years, there has been high interest in renewable energy and highly efficient devices, promoted by the need to stop changing weather patterns. One of the most interesting methods for this is using thermoelectric materials, which are low cost and highly durable. However, the need for higher efficiency values and a higher resistance to oxidation leads to a technological problem in the field of coating. Due to its diverse properties, glass coating has been proposed as a solution to both sublimation of the thermoelectric materials and oxidation. Lead silicate glasses with 30% PbO were doped with 0–5% of Na2O and B2O3 to produce glasses with different properties. Differential scanning calorimetry and dilatometry measurements showed that the glass temperature can vary between 428 and 505 °C. The softening temperature is varied between 493 and 560 °C. Below Tg, the coefficient of thermal expansion is varied between 5.9 and 9 ppm/K and above Tg it varied between 17 and 58 ppm/K. This allows the tuning of the glass composition for each thermoelectric material, such as 0.5% B and 1% Na doped PbO -SiO2 glass for skutterudites and 1% doped B and 1% Na doped for Mg2Si, PbTe, and GeTe.