Article contents
Probing the influence of post-processing on microstructure and in situ compression failure with in silico modeling of 3D-printed scaffolds
Published online by Cambridge University Press: 27 July 2018
Abstract
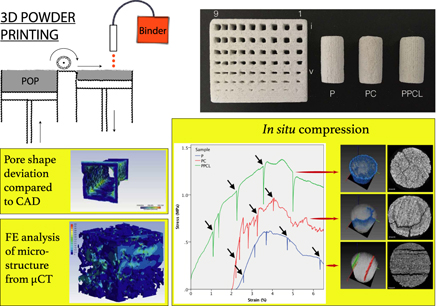
The post-processing treatment plays an important role in tailoring the mechanical and biological properties of the three-dimensional powder-printed porous scaffolds. Depending on scaffold material composition, a combination of post-processing treatments can be used to tailor these properties. This work probes into the impact of post-processing on the microstructure and deformation behavior of 3D-printed scaffolds. In this study, we have chosen CaSO4·xH2O (POP), a system for 3D powder printing and two different post-processing methodologies, namely chemical conversion and polymer infiltration. POP-based scaffolds were fabricated using water-based binder with up to 55% interconnected microporosity and moderate compressive strength of 1.5 MPa. Microcomputed tomography (µCT) is extensively utilized to determine the accuracy and efficacy of the adopted printing and post-processing approach. It was shown that the reproducibility of the fine features depends not only on the size but also on the presence of neighboring features. Crucially, µCT-based microstructure modeling and finite elemental simulation were attempted to computationally capture the compression behavior, in silico. Finally, in situ compression coupled with µCT imaging provided us an insight into fracture behavior of 3D powder-printed scaffolds.
Information
- Type
- Invited Article
- Information
- Journal of Materials Research , Volume 33 , Issue 14: Focus Issue: 3D Printing of Biomaterials , 27 July 2018 , pp. 2062 - 2076
- Copyright
- Copyright © Materials Research Society 2018
References
REFERENCES
- 9
- Cited by

