Article contents
Stability of amorphous Ta–O nanotubes prepared by anodization: Thermal and structural analyses
Published online by Cambridge University Press: 31 March 2014
Abstract
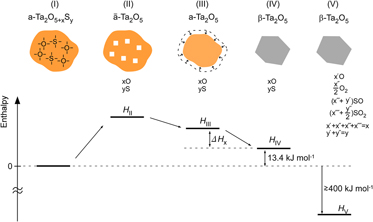
Amorphous Ta–O nanotubes (NTs) prepared by anodization in a sulfuric-acid-based solution have been found to contain considerable amounts of extra oxygen and sulfur. Their structural and thermal stability has been studied by combining x-ray diffractometry, transmission electron microscopy, and thermal analysis. The amorphous Ta–O, whose composition was estimated to be Ta2O6.6S0.7, crystallizes into orthorhombic β-Ta2O5 at temperatures around 1073 K by an endothermic reaction, at which excess oxygen and impurity sulfur are released. The amorphous NTs were found to be thermally more stable than stoichiometric amorphous Ta2O5, whose crystallization temperature is around 973 K. Excess oxygen and impurity sulfur, which form chemical bonds with Ta atoms in the amorphous solid, must be the origin of the stability. The crystallization follows the out-diffusion of oxygen and sulfur from the solid at temperatures where the mobility of atoms is high enough, indicating that the crystallization is kinetically arrested.
Information
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2014
References
REFERENCES
- 6
- Cited by

