Introduction
Electrospraying is a versatile technique that enables the facile synthesis of nanoparticles. While electrospraying has typically been used for the production of polymeric nanoparticles, it has also recently garnered some interest for the synthesis of ceramics, specifically the use of electrospray for the deposition of ceramic films [Reference Fantini, Zanetti and Costa1, Reference Jaworek2, Reference Jaworek3, Reference Luo, Okubo, Nangrejo and Edirisinghe4, Reference Sigmund, Yuh, Park, Maneeratana, Pyrgiotakis, Daga, Taylor and Nino5, Reference Yuh, Nino and Sigmund6, Reference Yuh, Perez, Sigmund and Nino7, Reference Yuh, Perez, Sigmund and Nino8]. This may be in part due to the difficulty of obtaining discrete ceramic particles using electrospraying rather than films of the ceramic material. This work develops a method that overcomes the limitation of traditional electrospraying, by electrospraying cobalt ferrite sol–gel precursor solutions into a liquid collector. This modified electrospraying platform enables the facile and scalable synthesis of nanoparticles.
Electrospraying uses an electric field that is created between a grounded collector and a syringe nozzle to shear a liquid jet into droplets [Reference Jaworek and Sobczyk9]. In a typical electrospray setup, a metal syringe needle is connected to a high-voltage power supply while a copper collecting plate is connected to the ground. By applying a voltage to the syringe needle, an electric field is created between the syringe and the collector plate. The syringe is placed in a syringe pump, and as the syringe is compressed, a droplet of the precursor solution is formed at the needle tip. Because of the high voltage applied to the syringe needle, charges build up on the surface of the pendant droplet at the needle tip. The repulsion between like charges on the droplet surface combined with the Coulombic forces of the electric field causes a distortion of the droplet into a cone shape, referred to as the Taylor cone [Reference Li, Marquez and Xia10]. For electrospraying to occur, the repulsion of the charges on the surface of the droplet must overcome the surface tension of the droplet. At this point, a jet of solution is extruded into the electric field. In electrospraying, the lack of a viscoelastic restoring force (typically imparted to sol–gel ceramic precursor solutions by the presence of a polymer binder) results in the jet breaking up into droplets [Reference Jaworek and Sobczyk9, Reference Li, Marquez and Xia10, Reference Li and Xia11]. These charged droplets are propelled toward the collector plate by the electric field. During this process, the droplets are continually breaking up into smaller droplets, based on a balancing act between the surface tension and the charge in the droplet, until they solidify. Solidification in sol–gel electrospraying is driven by evaporation of the electrospraying solvent as well as the hydrolysis and condensation reactions of the sol–gel precursors.
Electrospraying, especially sol–gel electrospraying, is sensitive to a variety of solution properties and experimental parameters. Specifically, several of the solution properties of import are the viscosity, metal ion concentration, surface tension, and conductivity. Some of the experimental parameters that affect electrospraying are the flow rate of the solution, the applied voltage, the distance between the nozzle and the collector, the type of collector, and the humidity of the electrospray environment. This work focuses on altering the conductivity of the electrospraying solutions and how the sol–gel solutions react to the environmental humidity by the addition of chelating agents as well as focusing on how changing the type of collector can help in achieving electrosprayed magnetic nanoparticles.
Several challenges arise for sol–gel–based electrospraying, including the high conductivity of the complex oxide precursor solutions (approximately 3.84 mS/cm for 0.7 M cobalt ferrite precursors dissolved in ethanol) as well as the reactivity of the sol–gel solutions to water vapor in the electrospinning environment. One approach to modifying the solution conductivity is by changing the concentration of the metal ions in solution. By decreasing the concentration of the metal ions in solution, the conductivity of the solution can be reduced. However, the applicability of this approach is limited because sufficient metal ions in solution are required to form the desired product. Another approach to modify solution conductivity is to use less conductive solvents (i.e., ethylene glycol with a conductivity of 1.07 µS/cm in place of ethanol which has a conductivity of 30 µS/cm7) can help reduce the conductivity of the solution, making it easier to electrospray. Although both of these methods decrease the conductivity of the precursor solutions, the precursor solutions for electrospraying ceramic particles are still significantly more conductive than the solutions used to electrospray polymer particles (e.g., 0.868 µS/cm for 0.00006 M polyvinylpyrrolidone (1,300,000 MW) in ethanol) [Reference Nartetamrongsutt and Chase12].
In addition to challenges associated with solution conductivity, additional considerations are required for sol–gel electrospraying because of the sensitivity of the sol–gel precursors to water. Sol–gel synthesis involves the hydrolysis and condensation reactions of the precursors, where the presence of water can significantly affect the rate at which these reactions occur. The chemical equations below show the hydrolysis and condensation reactions for cobalt ferrite precursor solutions, where the presence of water in both the hydrolysis and condensation reactions can be seen. Altering the amount of water in either reactions can drive the reaction toward hydrolysis or inhibit condensation [Reference Sajjia, Oubaha, Prescott and Olabi13].
In reactions with metal alkoxides, the hydrolysis reaction creates metal–hydroxo complexes. This is followed by condensation reactions that form metal–oxo–metal bridges. In the case of sol–gel electrospraying, the source of water that drives the hydrolysis and condensation reactions is the water vapor in the electrospraying environment. The rate at which the hydrolysis and condensation reactions occur affects the size and morphology of electrosprayed ceramic particles, as well as whether or not particles can be achieved instead of films of the sprayed materials [Reference Li, Marquez and Xia10]. If these reactions happen quick enough during electrospraying, the droplets gel preventing the continuation of droplet breaks up in the field [Reference Li, Marquez and Xia10]. Thus, given that the humidity of the electrospraying environment affects the rate of sol–gel reactions, it can drastically affect the success of sol–gel electrospraying.
To mitigate some of the adverse effects of humidity on sol–gel precursor solutions, chelating agents can be added, which will help slow down condensation reactions as it hinders –OH groups from reacting with the metal ions [Reference Brinker and Scherer14, Reference Sanpo, Berndt, Ang and Wang15]. Chelating agents also aid in the formation of a homogeneous network of the metal ions, which helps in creating the desired end product. A variety of chelating agents have been demonstrated for the sol–gel synthesis of ceramic films or particles. Two particularly promising chelating agents for the synthesis of magnetic films and particles include citric acid and ethylene glycol [Reference Brinker and Scherer14, Reference Sanpo, Berndt, Ang and Wang15, Reference Garcia-Cerda, Rodriguez-Fernandez, Resendiz-Hernandez and García-Cerda16, Reference Gopalan, Joy, Al-Omari, Kumar, Yoshida and Anantharaman17]. For example, Sanpo et al. have shown that the addition of citric acid as a chelating agent for cobalt ferrite led to increased homogeneity of the sol–gel precursor solution and to a lower concentration of impurity oxides (e.g., cobalt oxide, magnetite, and maghemite) [Reference Sanpo, Berndt, Ang and Wang15]. They attribute the increased homogeneity of the sol–gel to the coordination of the metal ions with citric acid, which slows down condensation reactions by isolating the metal ions from one another [Reference Sanpo, Berndt, Ang and Wang15]. Ethylene glycol is another commonly used chelating agent in sol–gel syntheses and is often used in tandem with acidic chelating agents, such as citric acid, polyacrylic acid, or nitric acid [Reference Garcia-Cerda, Rodriguez-Fernandez, Resendiz-Hernandez and García-Cerda16, Reference Gharagozlou18, Reference Khosravi, Yazdanbakhsh, Goharshadi and Youssefi19, Reference Vivekanandhan, Venkateswarlu and Satyanarayana20]. The combination of ethylene glycol with some of these additional chelating agents has been shown to reduce the temperature at which crystalline ceramics are formed [Reference Vivekanandhan, Venkateswarlu and Satyanarayana20]. Therefore, the choice to use two chelating agents for sol–gel electrospraying precursor solutions could potentially enable the formation of crystalline particles with a low-temperature heating step.
In addition to changing the chemistry of the solutions, altering the methods of collection of electrosprayed particles could also aid in the successful electrospray synthesis of magnetic nanoparticles. The desire to alter the collection method for electrospraying ceramics stems from the tendency to obtain films of materials during electrospraying onto a flat plate collector rather than discrete particles. Thus, instead of a flat plate collector, the use of a liquid collection medium was developed here for its use in ceramic electrospraying. The liquid collection medium in electrospinning or electrospraying avoids the gelling of the as-sprayed particles into films as happens with electrospraying onto a flat plate collector.
The area of polymeric electrospinning and electrospraying that has thus far found the most benefit from the use of liquid collectors is that of the synthesis of nanofibers of natural polymers, such as cellulose and chitin [Reference Barber, Griggs, Bonner and Rogers21, Reference Quan, Kang and Chin22, Reference Zheng, Miao, Maeda, Frey, Linhardt and Simmons23]. For these materials, liquid collector baths are of great use because of the insolubility of the polymers in typical electrospinning solvents. Therefore, instead of using traditionally used organic solvents to dissolve these polymers, they are dissolved in an ionic liquid before electrospinning. Because of the nonvolatile nature of these ionic liquids, they cannot be used to electrospin onto a flat plate collector [Reference Quan, Kang and Chin22]. To overcome this limitation, these polymers can be electrospun into liquid baths composed of a nonsolvent for these polymers that is also miscible with the ionic liquid. When the electrified jet hits the nonsolvent-based liquid bath, a solid fiber is formed, thus enabling the electrospinning of natural polymer nanofibers with limited solubility in volatile solvents. Because the ionic liquid is miscible in the collection medium, this facilitates the removal of the ionic liquid during collection without requiring additional purification steps [Reference Barber, Griggs, Bonner and Rogers21, Reference Quan, Kang and Chin22, Reference Zheng, Miao, Maeda, Frey, Linhardt and Simmons23]. Here, we seek to expand this concept to overcome the challenges of forming films when cobalt ferrite nanoparticles coalesce on a flat collector plate. Unlike the use of ionic liquids and liquid collecting baths for natural polymers, this work uses a collection bath that is immiscible with the electrospraying solvent combined with heating of the collection bath to obtain discrete particles and to boil off the electrospraying solvent. The ability to maintain discrete particles, as can be achieved with this method of electrospraying, is important for scaling up of the synthesis to achieve high yields of the nanoparticles.
An additional benefit of switching from a flat plate collector to a liquid collector is the relative ease of scale-up. The maintenance of discrete particles, as can be achieved with electrospraying into a liquid collector, is necessary to scale-up the synthesis without decreasing the quality of the particles. In addition, with the method of electrospraying into a liquid collector, one relatively simple way to increase the yield is to electrospray with multiple spinnerets, which is a method that is frequently used in electrospinning to increase yield or to create composites of different types of electrospun fibers [Reference Dosunmu, Chase, Kataphinan and Reneker24, Reference Persano, Camposeo, Tekmen and Pisignano25, Reference Zhou, Gong and Porat26].
Spray pyrolysis is another method that has been developed for the large-scale synthesis of ceramic particles. In spray pyrolysis, an aerosol is formed from precursor solution, where the size and morphology can be controlled by the concentration of the precursors and the velocity of the generated droplet [Reference Okuyama and Wuled Lenggoro27]. Here, we present an electrospray-based synthesis of complex oxide particles that is a complementary alternative to spray pyrolysis. Using electrospinning of fibers, a variety of morphologies can be obtained by changing the configuration of the metal spinneret, allowing the realization of complex microstructures including Janus and core–shell [Reference Andrew, Starr and Budi28]. Thus, methods to fabricate ceramic nanoparticles and nanocomposites via electrospraying have the potential to provide an opportunity for synthesizing functional composites on a single nanoparticle. The electrospray method developed here uses a liquid collector, which enables maintaining discrete particles separate during the synthesis, which also has the potential for scaling up to a continuous flow reactor design.
Results and discussion
Figure 1 shows scanning electron micrographs (SEMs) of cobalt ferrite particles sprayed onto a flat plate collector, resulting in the formation of a film as the deposited particles get agglomerated and gelled. This film formation limits the ultimate yield of the synthesis using a flat plate collector. To overcome this limitation, we instead developed a method where the cobalt ferrite particles were electrosprayed into a liquid collector, where they would be dispersed, thereby avoiding agglomeration and remaining as discrete particles. Here, cobalt ferrite precursor solutions were electrosprayed into silicone oil (η = 1000 cSt), which was heated to 200 °C. The process of electrospraying into silicone oil at 200 °C was motivated by the work by Caruntu et al., where they obtained crystalline metal ferrite particles in diethylene glycol heated to 220 °C [Reference Caruntu, Remond, Chou, Jun, Caruntu, He, Goloverda, O'Connor and Kolesnichenko29]. In addition to the potential to promote the formation of crystalline particles during the electrospray synthesis, heated silicone oil also allowed for the solidification of the droplets via the sol–gel combustion of the nitrates (oxidant) and citric acid (chelator and fuel) [Reference Caruntu, Remond, Chou, Jun, Caruntu, He, Goloverda, O'Connor and Kolesnichenko29]. This reaction began the formation of crystalline cobalt ferrite and removal ethylene glycol from the droplets. The solid particles can then be collected via centrifugation and washing. Figure 2 shows the X-ray diffraction (XRD) spectra for the as-sprayed and calcined particles. For the nanoparticles as-sprayed into 200 °C silicone oil, XRD showed that while there was some crystallinity present, a high-temperature calcination step was necessary to achieve fully crystalline nanoparticles.
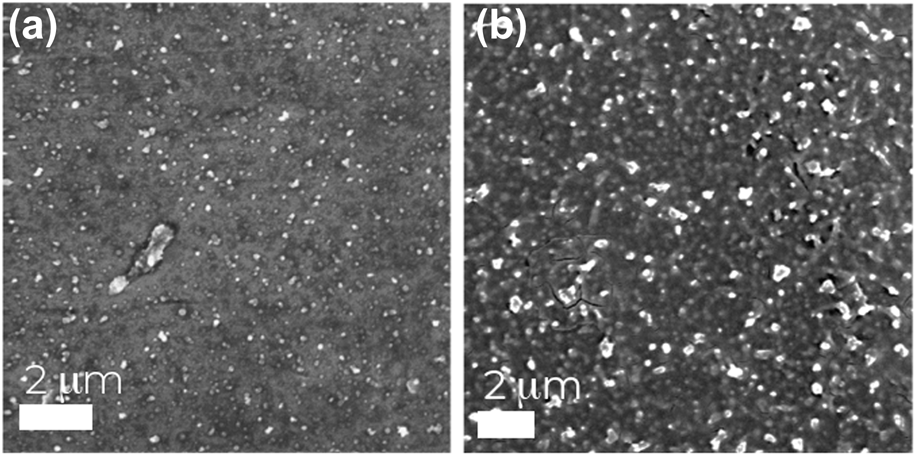
Figure 1 (a-b): Scanning electron microscope (SEM) images of films formed from electrospraying cobalt ferrite particles onto a flat plate collector.

Figure 2: Characterization of the composition of the cobalt ferrite particles. (a) XRD data of both as-sprayed and salt calcined nanoparticles confirming the presence of cobalt ferrite and (b) Raman spectroscopy showing peak characteristics of cobalt ferrite.
A salt calcination step was performed at 800 °C to crystallize the nanoparticles, while also preventing agglomeration, where the salt provides a physical barrier to prevent the particles from sintering together. A significant volumetric excess of salt to particles was used to ensure that the particles were isolated from another to avoid sintering. A calcination temperature of 800 °C was chosen based on a previous work in our group for electrospinning cobalt ferrite nanofibers, where we found that highly crystalline cobalt ferrite with good magnetic properties can be obtained for calcination temperatures spanning 650–1100 °C [Reference Starr and Andrew30, Reference Starr, Budi and Andrew31, Reference Budi, Glass, Rudawski and Andrew32, Reference Bauer, Wen, Tiwari, Arnold and Andrew33]. XRD (Fig. 2) confirms that the particles are crystalline following salt calcination. In addition, XRD confirmed the cobalt ferrite particles exhibit the inverse spinel structure. Scherrer's formula was applied to the XRD spectra to calculate an average crystallite size of 18.02 ± 0.04 nm for the cobalt ferrite particles.
Raman spectroscopy was also used to confirm the local ordering of the cobalt ferrite particles [Reference Sanpo, Berndt, Ang and Wang15]. Raman spectroscopy [Fig. 2(b)] shows peaks (indicated with red stars) at 688, 600, 467, and 315 cm−1, which corresponds to the cubic inverse-spinel nature of cobalt ferrite [Reference Da Silva, Melo, Soler, Lima, Da Silva and Morais34]. Raman spectroscopy confirms that the particles are composed of cobalt ferrite and lack impurities such as cobalt oxide or magnetite [Reference Sanpo, Berndt, Ang and Wang15].
Figure 3 shows SEM images for both as-sprayed and salt-calcined cobalt ferrite particles. The as-sprayed particles are surrounded by residual silicone oil that was not fully removed in the washing steps. Figure 3(b) shows the salt-calcined cobalt ferrite particles. However, to better resolve the individual particles, transmission electron microscopy (TEM) was performed. Figure 4 shows a representative TEM image of the calcined cobalt ferrite particles. The particle size measured from TEM images was found to be 20.7 ± 11.5 nm as determined from measurements of 500 particles, which is in good agreement with the value of 18.02 ± 0.04 nm obtained from Scherrer's formula. TEM shows that the particles remained discrete throughout the salt calcination process, highlighting that salt calcination provides a method that keeps them separate during calcination.
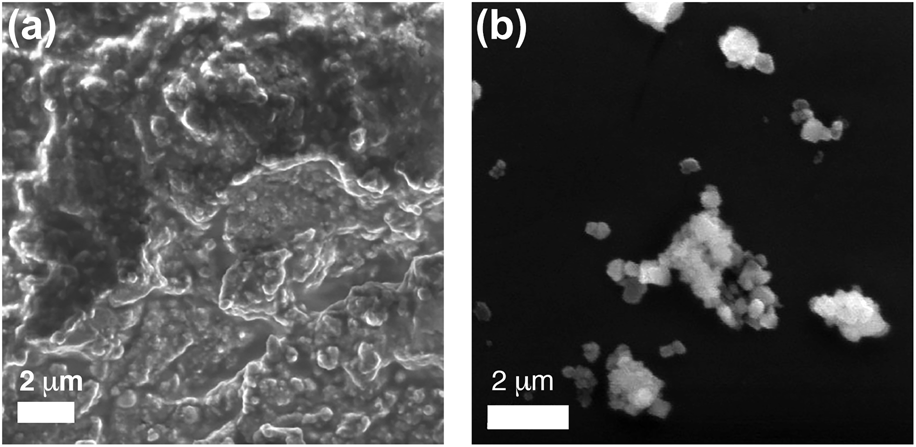
Figure 3: SEM images of (a) as-sprayed and (b) salt-calcined cobalt ferrite particles. The as-sprayed nanoparticles reveal that some silicone oil remains even after washing.
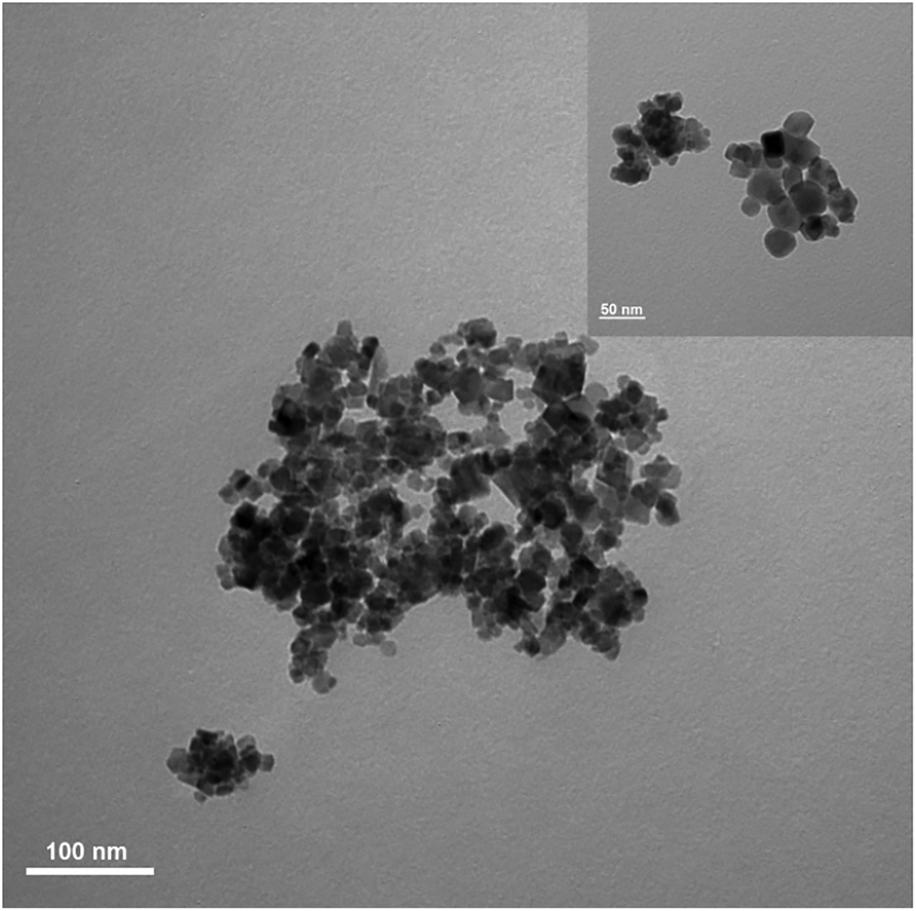
Figure 4: TEM image of salt-calcined cobalt ferrite nanoparticles.
The particles reported above all had a 2:1 molar ratio of Fe:Co nitrate–based precursors that were dissolved in ethylene glycol with 0.76 mmol of citric acid, which acted as a chelating agent. These solutions had a conductivity of 2.834 mS/cm and a viscosity of 12.5 cP. In addition, precursor solutions were prepared in ethylene glycol, but without the presence of citric acid, which resulted in a lower conductivity of 2.161 mS/cm, but the viscosity remained the same at 12.5 cP. However, the solutions that did not have citric acid tended to form agglomerated clusters of particles (Fig. 5). Citric acid chelates with the metal ions, resulting in a more homogeneous sol, while also limiting the condensation reaction and reaction between particles, preventing agglomeration.
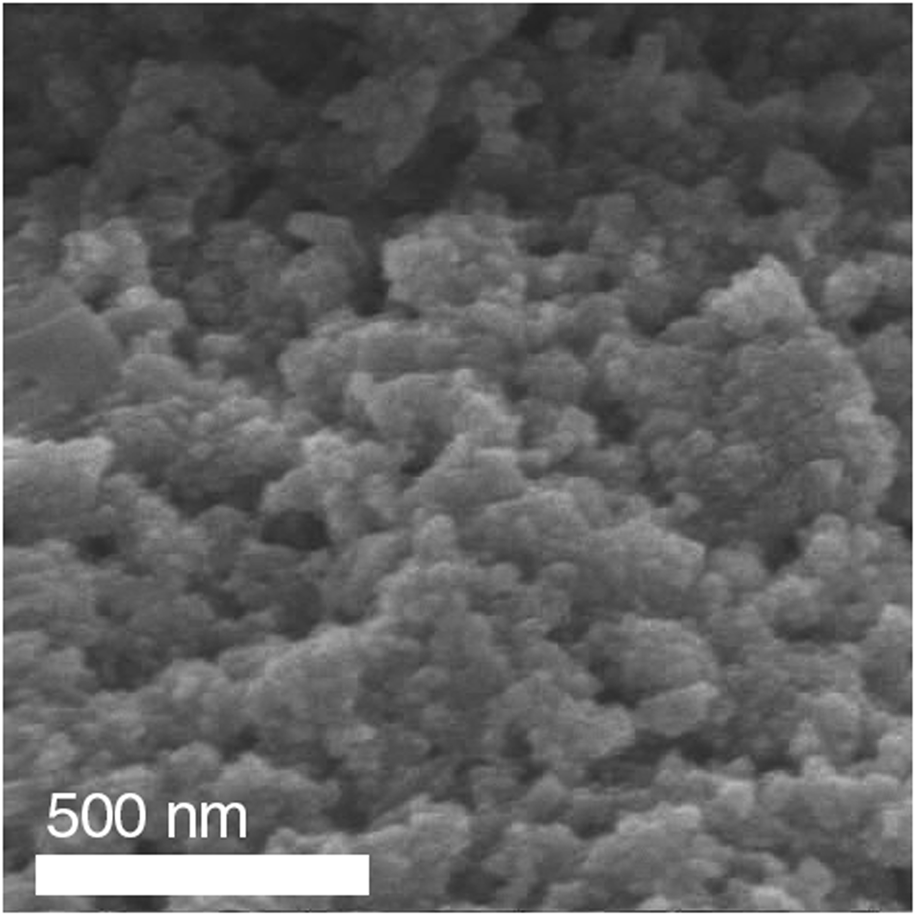
Figure 5: SEM image of calcined cobalt ferrite nanoparticles electrosprayed in the absence of the citric acid chelating agent.
SQUID magnetometry was used to examine the magnetic properties of the calcined cobalt ferrite nanoparticles. Room temperature hysteresis measurements revealed that the particles have a saturation magnetization of 75.3 ± 0.5 emu/g (Fig. 6). By comparison, the bulk saturation magnetization for cobalt ferrite is 80.8 emu/g [Reference Sanpo, Berndt, Ang and Wang15, Reference Meng, Li, Chen, Mei, Wang and Li35]. The lower room temperature saturation magnetization of the cobalt ferrite particles can be attributed to surface spin disorders caused by broken bonds on the surface of the nanoparticles [Reference Khurshid, Li, Phan, Mukherjee, Hadjipanayis and Srikanth36, Reference Tung, Kolesnichenko, Caruntu, Chou, O'Connor and Spinu37]. This results in what is often referred to as a “dead” layer on the surface of particles that does not contribute to the magnetization of the particles [Reference Unni, Uhl, Savliwala, Savitzky, Dhavalikar, Garraud, Arnold, Kourkoutis, Andrew and Rinaldi38]. The difference between the crystallite diameter of the cobalt ferrite (18.02 ± 0.004 nm) and the physical diameter as measured from TEM (20.7 ± 11.5 nm) could also be explained by this phenomenon.
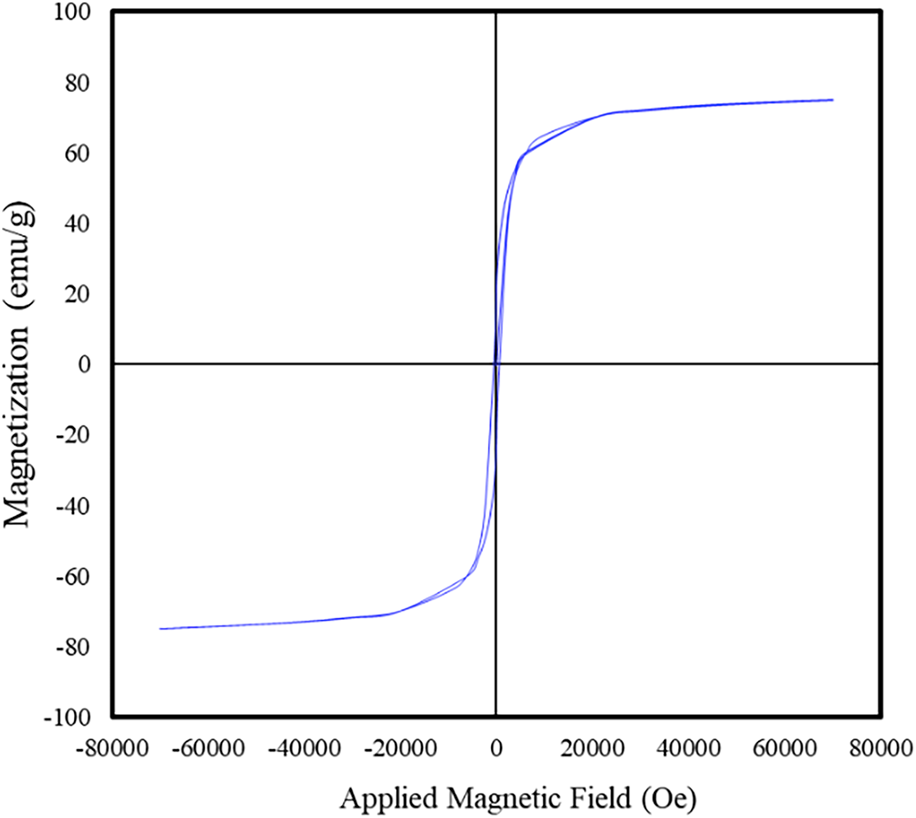
Figure 6: Room temperature hysteresis loop of the cobalt ferrite particles with an average saturation magnetization of 75.3 ± 0.5 emu/g.
Conclusions
A versatile electrospraying method, with the potential for high yield has been demonstrated for cobalt ferrite nanoparticles. By making use of a liquid collector, it was possible to electrospray cobalt ferrite precursor droplets that remained as individual droplets rather than congealing to form one large droplet. The ability to maintain discrete particles using this method makes it amenable to scale up for larger synthesis of magnetic nanoparticle. This method for electrospraying is worthwhile as it presents an opportunity for the development of functional composites within a single nanoparticle. With the newfound ability to produce single-phase ceramic nanoparticles via electrospraying, it opens the door to combine the methods used for creating composite ceramic nanofibers, such as core–shell or Janus ceramic electrospinning, and the methods presented here for the synthesis of ceramic nanoparticles via electrospraying easily create core–shell or Janus nanoparticles of functional ceramics via electrospray.
Experimental methods
Cobalt(II) nitrate hexahydrate, citric acid monohydrate, and 1000 cSt silicone oil were obtained from Sigma Aldrich. Ferric nitrate nonahydrate and ethylene glycol were obtained from Fisher Scientific. All chemicals were used without further modification.
To create sol–gel precursor solutions for cobalt ferrite electrospraying, 2.2 mmol of ferric nitrate nonahydrate and 1.1 mmol of cobalt(II) nitrate hexahydrate were dissolved in 3 mL of ethylene glycol. In addition, 0.76 mmol of citric acid monohydrate was dissolved in the ethylene glycol solution. The precursor solutions were mixed for two hours via magnetic stirring before electrospraying. Before electrospraying, the conductivity of the precursor solution was measured to be 2.83 mS/cm (∼1 mS/cm lower than solutions of Co and Fe nitrates in ethanol at the same concentration).
A schematic showing the setup used for electrospraying into the silicone oil collection medium is shown in Scheme 1. This work used a liquid collector, specifically 1000 cSt silicone oil to collect the as-sprayed cobalt ferrite particles. The silicone oil was heated to 200 °C using a hot plate to solidify the particles as they were collected. The temperature of the oil was monitored using a thermocouple until the desired temperature was reached. First, the silicone oil collector dish was placed on a grounded piece of aluminum foil on top of a hot plate, indicated in Scheme 1, by the grounded stir plate. The sol–gel precursor solution was then loaded into a syringe with a metal needle. The syringe was placed into a syringe pump and arranged at a distance of 22 cm from the surface of the silicone oil and was connected to a high-voltage source. A voltage of 11 kV was applied to the syringe needle, and the precursor solution was pumped at a flow rate of 0.15 mL/h. During electrospraying, the silicone oil was stirred to further ensure that the as-sprayed particles did not have the opportunity to coalesce before solidifying. Typical electrospraying experiments run for 8 h.
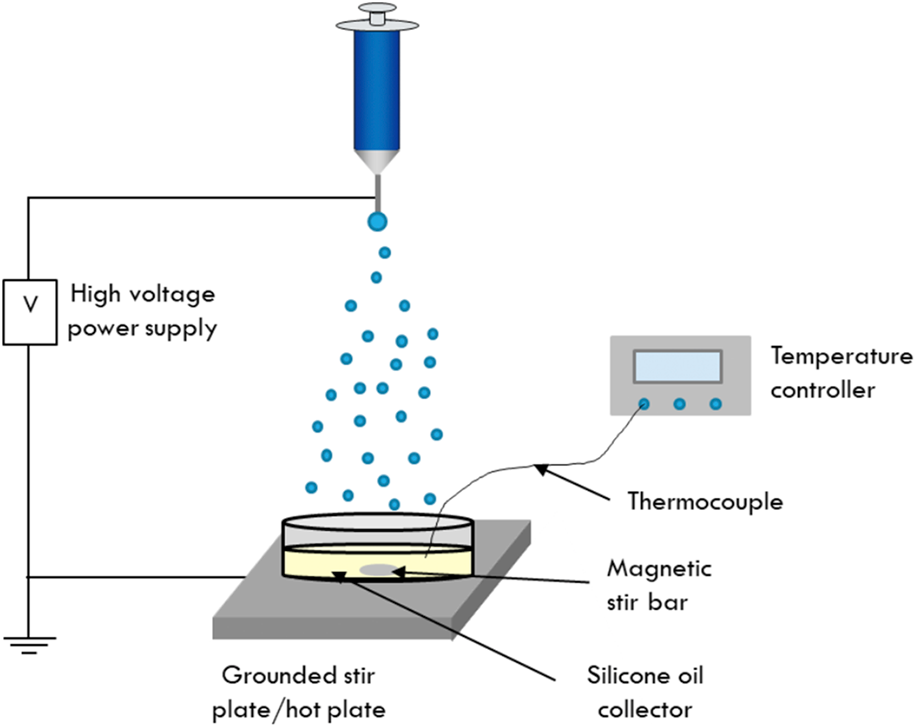
Scheme 1: Schematic of the electrospray setup developed for the synthesis of cobalt ferrite nanoparticles. Here, the particles are electrosprayed into a dish of silicone oil that is heated to 200 °C and magnetically stirred to maintain individual particles during the electrospray process.
After electrospraying, the silicone oil was allowed to cool to room temperature and was then thinned out using hexane. To collect the particles, this suspension was then centrifuged at 5000 rpm for 10 min. The supernatant was decanted, and the particles were rinsed additional five times in hexane to ensure the removal of the silicone oil. Following the hexane rinses, the particles were rinsed three times in toluene and three times in ethanol to remove both the hexane and toluene, respectively. The particles were then dried in a vacuum oven overnight before calcination.
To obtain crystalline nanoparticles, the as-sprayed particles were calcined using a salt calcination technique. This process involves grinding sodium chloride into fine powder using a mortar and pestle. The washed and dried as-sprayed particles were then mixed into the salt powder using a mortar and pestle, where the volumetric ratio between sodium chloride and the cobalt ferrite nanoparticles was at least 500:1. The mixture was then heated to 800 °C at a ramp rate of 10 °C/min and held for 3 h. Once cooled to room temperature, the salt was dissolved in deionized water, and the suspensions were centrifuged using 100,000 NMWL Amicon Ultra-15 centrifugal filters. After filtering, the particles were rinsed three additional times with deionized water to ensure complete removal of the salt. The particles were then dried in a vacuum oven overnight before characterization.
Scanning electron microscopy (SEM; FEI Nova 430) was used to analyze the morphology of the as-sprayed and salt-calcined cobalt ferrite particles. Transmission electron microscopy (TEM; Hitachi, H7000 100 keV) was used to further examine the nanoparticle size and morphology. ImageJ was used to analyze TEM images to obtain an average particles size, where 500 nanoparticles were measured to determine an average particle size. X-ray diffraction (XRD; PANalytical X'Pert Powder) was used to confirm that the particles were composed of cobalt ferrite. Scherrer's formula, in conjunction with the XRD data, was used to calculate the crystallite size of the cobalt ferrite particles. Raman spectroscopy (Renishaw, Invia Raman Microscope) was also used to analyze the composition of the nanoparticles and ensure the absence of other oxides of cobalt or iron. Finally, the magnetic behavior of the cobalt ferrite particles was examined using a superconducting quantum interference device (SQUID magnetometry) (Quantum Design MPMS 3).
Acknowledgment
This work was supported by the National Science Foundation through a CAREER Award (DMR-1150665).










