Article contents
Synthesis of multi-branched gold nanostructures and their surface-enhanced Raman scattering properties of 4-aminothiophenol
Published online by Cambridge University Press: 07 February 2019
Abstract
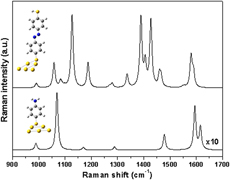
A facile one-pot and environmentally friendly method was developed to synthesize multi-branched flowerlike gold (Au) nanostructures by reducing chlorate gold (HAuCl4) with hydrogen peroxide (H2O2) in the presence of sodium citrate. The multibranched Au nanostructures were characterized by transmission electron microscopy and Ultraviolet-visible (UV-vis) absorption spectroscopy. The molar ratio of sodium citrate to HAuCl4 and the concentrations of the reacted reagents play important roles in the formation of multibranched Au nanostructures. The multibranched Au nanostructures with sharp tips exhibit excellent surface-enhanced Raman scattering (SERS) ability of 4-aminothiophenol (PATP). The experimental and simulated results both confirm that the photoinduced catalytic coupling reaction of PATP transformation to 4,4′-dimercaptoazobenzene occurs on the surface of multibranched Au nanostructures at a high power during the SERS measurement. It is believed that these multibranched Au nanostructures may find potential applications in SERS, biosensors, and the photoinduced surface catalytic application fields.
Keywords
Information
- Type
- Article
- Information
- Journal of Materials Research , Volume 34 , Issue 17: Focus Issue: Building Advanced Materials via Particle Aggregation and Molecular Self-Assembly , 16 September 2019 , pp. 2928 - 2934
- Copyright
- Copyright © Materials Research Society 2019
References
- 7
- Cited by

