Published online by Cambridge University Press: 31 January 2011
Two materials of α-type manganese dioxide were synthesized and examined. They were prepared by the pyrolysis of mixtures of MnCO3 and (CH3)3COK. An ill-ordered material was obtained when prepared at large (CH3)3COK content. Both samples behave as acids, but their apparent capacities are obviously different: about 0.9 meq/g for a well-ordered sample and about 2.6 meq/g for an ill-ordered sample. Ion-exchange properties were examined on Kielland's plot. Zero intersections of the two samples are almost the same 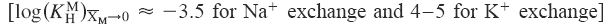 but slopes are different (about −50 for a well-ordered sample and about −10 for an ill-ordered sample). The difference in slope is likely caused by the flexibility. An evidence of the flexibility can be seen by x-ray diffractometry.
but slopes are different (about −50 for a well-ordered sample and about −10 for an ill-ordered sample). The difference in slope is likely caused by the flexibility. An evidence of the flexibility can be seen by x-ray diffractometry.