As life expectancy increases, so too does the number of individuals with dementia and cognitive decline. In 2015, there were an estimated 46·8 million adults living with dementia worldwide, with a new case of dementia diagnosed every 3·2 s(1). Unfortunately, there are currently few effective treatments once a dementia diagnosis is made. Thus, nutrition interventions to reduce the prevalence of cognitive decline are of great interest to public health.
Decreases in cognitive function related to increases in oxidative stress commonly occur with ageing. High rates of energy production required to maintain the central nervous system subject the brain to risk of oxidative imbalance, leading to neuro-inflammation(Reference Glade2). Although reactive oxygen species (ROS) serve as signalling molecules at physiological levels, oxidative stress ensues when ROS production exceeds homoeostatic mechanisms to detoxify. The accumulation of ROS along with oxidised protein, lipid and nucleic acids from prolonged oxidative imbalances damages neurons and myelin sheaths. Furthermore, chronic oxidative stress in the brain leads to neuro-inflammation, a hallmark feature of cognitive ageing and dysfunction along with neurodegenerative diseases(Reference Glade2, Reference Mittal and Katare3). In contrast to the deleterious effects of oxidative imbalance on cognition, systemic oxidative balance may positively influence cognitive longevity or maintained cognitive performance.
Fruit and vegetables contain numerous bioactive compounds exhibiting antioxidant and anti-inflammatory functionality, such that their intake may have protective effects against cognitive decline by maintaining and restoring oxidative and inflammatory balance. According to a recent meta-analysis of nine studies (n 31 104), increased consumption of fruit and vegetables was associated with significant reduction in the risk of cognitive impairment and dementia (OR = 0·87, 95 % CI 0·77, 0·99)(Reference Jiang, Huang and Song4); however, equivocal findings were reported in a recent systematic review of the literature reporting on associations between cognitive function or dementia and intake of specific antioxidants in fruit and vegetables including β-carotene, vitamins C and E and flavonoids(Reference Crichton, Bryan and Murphy5). In contrast, higher intake of the bioactive carotenoid lutein has been linked to better cognitive performance(Reference Johnson6). Although the exact mechanisms of the neuroprotective activity of lutein are unknown, the beneficial effects of this carotenoid compound are thought to be attributable to its ability to decrease oxidative stress. Divergent associations between carotenoids, specifically β-carotene and lutein, and cognitive function or dementia may be the result of differences in tissue distribution of these carotenoids as well as differing antioxidant functionality. For example, lycopene, a carotenoid present in several fruits including tomatoes and watermelon, has demonstrated greater singlet oxygen quenching abilities compared with the other carotenoids, such as β-carotene, lutein and zeaxanthin(Reference Di Mascio, Kaiser and Sies7). In recent studies using animal models, lycopene has been shown to exhibit neuroprotective effects by mitigating oxidative stress, suppressing production of inflammatory cytokines, and attenuating amyloidosis or accumulation of amyloid plaques(Reference Wang, Li and Wang8, Reference Liu, Wang and Yi9); furthermore, lycopene has been shown to attenuate cognitive deficits by improving inflammation in the gut–liver–brain axis as well improving glycolipid metabolism(Reference Wang, Wang and Li10). Additional mechanisms by which lycopene confers neurocognitive protection include the inhibition of neuronal apoptosis and restoration of mitochondrial function(Reference Chen, Huang and Chen11). To date, the current evidence on the relationship between lycopene and cognition in humans has yet to be synthesised.
As there are several stages of cognitive decline, so, too, are there several methods for assessing and subsequently diagnosing loss of cognitive function. For example, the term ‘dementia’ describes a very broad range of symptoms that may include loss of memory, language, focus, problem-solving and visual perception(12). Currently, Alzheimer's disease (AD) is the most common form of dementia worldwide(Reference Dias, Polidori and Li13). As such, the aim of the present review is to assess if there is evidence for a protective relationship between lycopene and maintained cognitive function as well as assess its relationship with the development and progression of dementia.
Methods
Literature search
A systematic literature search was conducted according to published guidelines for systematic reviews. Searches were conducted using PubMed and EBSCOhost. The selected databases offer different filters for search results. Filters for ‘peer reviewed’, ‘scholarly journals’, and ‘ages 65+ years’, ‘middle aged: 45–64 years’, ‘aged (65 years & older)’, ‘aged 80 & over’, ‘very old (85 years & older)’ and ‘middle aged (40–64 years)’ were included in the EBSCOhost search. Filters for human studies were placed on the PubMed search. Articles were included if published after 2005, with titles and abstracts in English, and in full text rather than as an abstract or presentation format only.
Application of inclusion/exclusion criteria
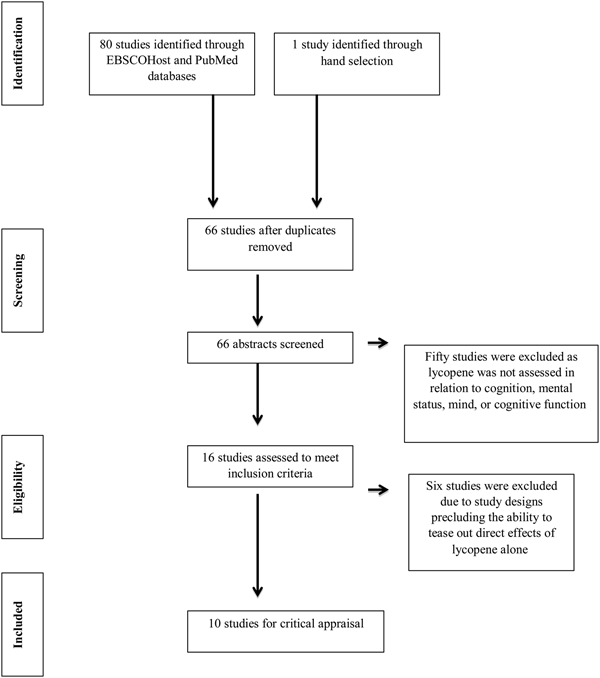
Boolean search terms were used to identify the primary articles to be considered for the review. Search terms for ‘lycopene’ and ‘cognition or mental status or brain function or cognitive function or memory or brain’ and ‘elderly or older adults or geriatric’ were applied. Identical searches were conducted using EBSCOhost and PubMed databases. Additionally, searches were also conducted using lycopene-rich foods such as tomatoes as search terms; however, no additional studies meeting the inclusion criteria were identified. One publication did not appear in search results or in the article references but was hand-selected due to its topical relevance to the objective of this study(Reference Feart, Letenneur and Helmer14). After duplicate articles were removed, sixty-six publications remained. The first author (K. M. C.-W.) screened articles identified in the searches by abstract and a keyword search of the full paper. Fifty articles were excluded as they did not assess lycopene in relation to ‘cognition’, ‘mental status’, ‘mind’ or ‘cognitive function’. The references of identified articles were also reviewed to check for any articles not identified by the Boolean searches; however, no new articles were identified for inclusion following abstract review by the first author. The review process is reported in Fig. 1.

Fig. 1. Search strategy flow diagram for research evaluating the relationship between lycopene and cognition.
Data extraction and analysis
To prevent risk of bias in reporting, the remaining sixteen articles were independently analysed by all authors for quality according to the Academy of Nutrition and Dietetics Evidence Analysis Library Worksheet and Quality Criteria Checklist(15). Following article review and data abstraction, six articles were excluded. Although the abstracts mentioned lycopene, the papers did not meet the inclusion criteria(Reference Niu, Guo and Kakizaki16–Reference Nilsson, Salo and Plaza21). One study only considered incidence of depression in participants rather than specific areas of cognitive functioning and was thus excluded for unrelated outcome measures(Reference Niu, Guo and Kakizaki16). Another article assessed mental health status, not cognition(Reference Wilkinson, Farrelly and Low18). The third reported on cognition and the Mediterranean diet as a whole rather than lycopene specifically(Reference Shahar, Houston and Hue17). The remaining three papers describe studies providing a combination supplement or a beverage that included lycopene together with other antioxidants(Reference Yasuno, Tanimukai and Sasaki19–Reference Nilsson, Salo and Plaza21). These were not included due to the inability to tease out any direct effects from lycopene alone.
Results
Study characteristics of research identified
Of the eighty-one articles that were identified and considered for the present review, ten articles fit the inclusion criteria (Table 1)(Reference Dias, Polidori and Li13, Reference Feart, Letenneur and Helmer14, Reference Akbaraly, Faure and Gourlet22–Reference Min and Min29). The articles were published between 2007 and 2015 from the USA, France, Germany, Scotland and Brazil. Of the ten studies, five had a cross-sectional study design(Reference Dias, Polidori and Li13, Reference Akbaraly, Faure and Gourlet22, Reference Polidori, Praticόc and Mangialasche23, Reference von Arnim, Herbolsheimer and Nikolaus27, Reference Giavarotti, Simon and Azzalis28) and five assessed longitudinal data with study duration lasting from 1·8 years to an average of 17 years(Reference Feart, Letenneur and Helmer14, Reference Devore, Kang and Stampfer24–Reference Whalley, Duthie and Collins26, Reference Min and Min29). These ten publications assessed lycopene and cognition in both men and women between the ages of 45 and 102 years.
Table 1. Results from studies assessing the relationship between lycopene and cognitive function

%F, percentage female; NR, not reported; TICS, Telephone Interview for Cognitive Status; AD, Alzheimer's disease; CERAD, Consortium to Establish a Registry for Alzheimer's Disease.
Discussion of the ten articles is divided into sections on the relationship between lycopene intake or circulating levels of lycopene and (1) cognitive performance, (2) development of dementia and (3) influence on pre-existing dementia.
Lycopene and cognitive performance
Of the ten articles included in this review, four focused on lycopene and maintenance of normal cognitive function at a single time point or the relationship between lycopene intake at one point and cognitive outcomes years later(Reference Akbaraly, Faure and Gourlet22–Reference Kesse-Guyot, Andreeva and Ducros25).
In a cross-sectional study by Akbaraly et al.(Reference Akbaraly, Faure and Gourlet22), low plasma lycopene was correlated with low cognitive performance in a cohort of 589 community-dwelling men and women aged 68–79 years. After adjustment for possible confounders, participants in the lowest percentile of cognitive function were more likely to have the lowest levels of circulating lycopene. When cognitive function tests were administered, low levels of lycopene were significantly associated with the Trail Making Test B (P = 0·008) and Digit–Symbol Substitution test (P = 0·01) (OR = 1·76 and 2·02, respectively).
In another cross-sectional analysis, Polidori et al.(Reference Polidori, Praticόc and Mangialasche23) examined plasma lycopene relative to cognition in a German cohort of 193 men and women aged 45–102 years. FFQ were used to group participants into ‘high’ or ‘low’ intake groups for fruit and vegetables. Participants in the ‘high intake’ group for fruit and vegetables had significantly higher plasma lycopene compared with those in the ‘low intake’ group (P < 0·0001). Specifically, higher plasma lycopene levels were associated with higher test scores on the Mini-Mental State Examination (MMSE) (P = 0·005), the Dem-Tect Scale (P = 0·02) and the Clock Drawing Test (P < 0·0001).
In the Nurses' Health Study, cognition was assessed in 16 010 female nurses aged 70 years and older using a battery of six cognitive tests that were repeated three times at 2-year intervals(Reference Devore, Kang and Stampfer24). The cognitive tests included the Telephone Interview for Cognitive Status (TICS) test, East Boston Memory Test – immediate and delayed recalls, a category fluency test, a delayed recall of the TICS ten-word list, and backward digit span test. The global score consisted of the average z scores of all six tests, and the verbal score included the immediate and delayed recalls of the East Boston Memory Test and the TICS ten-word list. Additionally, an average of five FFQ was given over approximately 17 years. The dietary assessments were collected in the years prior to cognitive testing and were assumed to depict the participants' usual intake before and during the cognitive assessment period. Lycopene intake was associated with a slower decline in both the global and verbal composite scores (P = 0·05, P = 0·009, respectively) over the course of the 6 years that the cognitive tests were administered. Results suggest a potential relationship between long-term consumption of foods high in lycopene and preserved cognitive function.
Another longitudinal study reported an association between carotenoid-rich dietary patterns and cognition later in life(Reference Kesse-Guyot, Andreeva and Ducros25). In this group of 2983 men and women aged 45–60 years, dietary data were obtained from twelve individual 24-h dietary recalls administered over 2 years. Plasma concentrations of lutein, zeaxanthin, β-cryptoxanthin, lycopene, α-carotene, trans-β-carotene and cis-β-carotene were measured at baseline for a subset of the participants (n 381). Cognitive function was assessed 13 years later by the RI-48 cued recall task, several verbal fluency tasks, the forward and backward digit span tests and the Trail Making Test. Carotenoid-rich diet patterns ascertained from 24-h recalls at baseline were correlated with total plasma carotenoid concentrations collected at the same time, although a statistically significant association with plasma lycopene specifically was not observed. Dietary patterns rich in total carotenoids over the first 2 years of data collection were associated with a higher composite score of cognitive function at the 13-year follow-up (P < 0·05); however, correlations between plasma lycopene at baseline and cognitive scores 13 years later were not reported. Thus, although this study does provide evidence for a relationship between higher intakes of carotenoid-rich foods and preserved cognitive function with advancing age, it does not provide evidence for the effects of lycopene specifically.
Lycopene and the development of dementia
Only two studies considered lycopene status and risk of dementia diagnosis later in life(Reference Feart, Letenneur and Helmer14, Reference Whalley, Duthie and Collins26), and both reported circulating lycopene levels rather than a self-reported measure of intake. Based on the relationship between high homocysteine and increased risk of AD, Whalley et al.(Reference Whalley, Duthie and Collins26) investigated circulating homocysteine, folate and vitamin B12 among a sample of 201 men and women born in 1921. Plasma lycopene was analysed among a subsample of 173 participants, and associations were examined between baseline blood levels of these circulating nutrients and development of dementia across 11 years. Dementia diagnosis was based on International Statistical Classification of Diseases and Related Health Problems (ICD)-10 criteria. Plasma lycopene, among several other antioxidant compounds, showed a significant inverse relationship with plasma homocysteine levels (P = 0·017); furthermore, it was reported that low plasma lycopene concentrations were associated with increased risk of dementia (P value not reported). Based on the analyses, it is unclear whether this was an independent risk or if it was associated with the risk of high homocysteine, given the inverse association between lycopene and homocysteine.
Feart et al.(Reference Feart, Letenneur and Helmer14) did not find significant inverse associations between plasma lycopene and dementia diagnosis in a longitudinal study of 1092 French men and women with an average age of 74·4 years. In this study, researchers assessed the relationship between plasma carotenoids (β-carotene, α-carotene, lycopene, lutein, zeaxanthin and β-cryptoxanthin) measured at baseline and the subsequent risk of developing dementia or AD. All participants were free of dementia at baseline, and cognitive status was assessed by a team of neurologists over the 10-year average follow-up period. It was determined that decreased risk of dementia and AD was associated only with higher lutein concentrations in the older adult participants (P < 0·05). Although circulating levels of lycopene were lower in participants later diagnosed with dementia compared with those without dementia, differences did not reach statistical significance.
Lycopene and pre-existing dementia
Four studies assessed lycopene among participants with pre-existing dementia(Reference Dias, Polidori and Li13, Reference von Arnim, Herbolsheimer and Nikolaus27–Reference Min and Min29). Each of the studies utilised a comparison group and reported circulating lycopene levels rather than a self-reported intake. Dias et al.(Reference Dias, Polidori and Li13) analysed blood samples from twenty-seven AD participants without cardiovascular co-morbidities (AD only), sixteen AD participants with concomitant cardiovascular co-morbidities (AD plus), and a control group of thirty-three healthy older adults. Among the primary outcome measures of this study were HDL-cholesterol concentrations, MMSE scores and plasma carotenoid concentrations (lycopene, β-carotene, α-carotene, lutein, zeaxanthin and cryptoxanthin). Participants with ‘AD plus’ had lower plasma concentrations of HDL-cholesterol (P < 0·05) and lycopene (P < 0·05) compared with the control and ‘AD only’ groups. Lycopene was not significantly lower in the ‘AD only’ group when compared with the control group. Based on the results, it is unclear if there is an inter-relationship between circulating lycopene, CVD and AD.
In a cross-sectional study of 222 German men and women aged 65–90 years, von Arnim et al.(Reference von Arnim, Herbolsheimer and Nikolaus27) investigated the association between mild dementia and serum antioxidants (vitamin C, vitamin E, β-carotene, lycopene, coenzyme Q10). Mild dementia was defined by an MMSE score < 24, and diagnosis was confirmed by a battery of neurological tests. According to these criteria, there were seventy-four men and women in the group designated as having mild dementia. The study enrolled 148 men and women who scored over 24 on the MMSE for an age- and sex-matched control group. Though descriptive findings suggest that individuals consuming more lycopene are at lower odds of having dementia compared with those that consume lesser amounts of lycopene, statistical findings from the adjusted models were not significant. Of concern, the authors do note, however, that ‘measurement error in the outcome variable’ of serum lycopene could have occurred.
In a cross-sectional study by Giavarotti et al.(Reference Giavarotti, Simon and Azzalis28), plasma lycopene levels in twenty-three elderly men and women with probable AD were compared with forty-two controls (mean age of all participants = 82 years). Cognitive status was assessed by MMSE and Clinical Dementia Rating scores. Although mean plasma levels of lycopene were lower in participants with probable AD compared with those with normal cognitive function, the difference was not significant.
Min & Min(Reference Min and Min29) evaluated existing data from the Third National Health and Nutrition Examination Survey (NHANES III) and the NHANES III Linked Mortality File to assess the relationship between serum carotenoids and risk of death from AD. The study population which included 6968 individuals over the age of 50 years was assessed at two cross-sectional time points. Serum carotenoids (α-carotene, β-carotene, β-cryptoxanthin, lycopene, lutein plus zeaxanthin) were measured at baseline (between 1988 and 1994), and deaths attributable to AD were quantified from ICD-9 and ICD-10 codes through the year 2000. Mortality data showed seventy-five deaths from AD over the follow-up period, and participants with AD mortality had significantly lower serum levels of lycopene compared with those without AD mortality (P < 0·0001). Results suggest a potential association between decreases in serum lycopene and death attributable to AD.
Discussion
The present review is the first of its kind to assess available research on the relationship between lycopene and cognition. A key discovery was the paucity of research in this area, especially of intervention studies. Among the four studies evaluating the relationship between circulating or dietary lycopene and maintained cognition, three reported significant positive relationships(Reference Akbaraly, Faure and Gourlet22–Reference Devore, Kang and Stampfer24). In contrast, neither of the two studies reporting on the relationship between lycopene and future dementia development reported significant results(Reference Feart, Letenneur and Helmer14, Reference Whalley, Duthie and Collins26). However, in both studies, plasma lycopene was lower in those with future dementia diagnosis albeit non-significantly. Lastly, among the four studies investigating circulating lycopene in older adults with pre-existing dementia, only one reported significantly lower circulating levels of lycopene among those with AD mortality matched to a comparison group(Reference Min and Min29). Taken collectively, four of the ten articles identified for inclusion in the present review reported statistically significant protective associations(Reference Akbaraly, Faure and Gourlet22–Reference Devore, Kang and Stampfer24, Reference Min and Min29). Nevertheless, the study designs preclude the ability to ascribe causality both due to potential confounding and a lack of knowledge about the temporal relationship between variables of interest.
Although functional MRI studies have associated plasma lycopene with enhanced cognitive performance, findings reported herein warrant additional discussion as several factors probably mediate the relationship between lycopene and cognition(Reference Zwilling, Talukdar and Zamroziewicz30). It must be acknowledged that lycopene accumulates differently in tissues, and this accumulation is influenced by both genetic factors and phenotypic influences(Reference Moran, Erdman and Clinton31, Reference Yeum and Russell32). Thus, inter-personal variability in the bioavailability and distribution of lycopene would be expected to influence its relationship with cognition. Furthermore, dietary factors such as the food matrix may also have a significant impact on the bioavailability of lycopene(Reference van het Hof, West and Weststrate33). For this reason, circulating levels of lycopene are superior to self-reported measures of dietary intake in assessing the relationship between lycopene and cognition. All but one study included in the present review included a circulating measure of lycopene, and more specifically, three of the four studies reporting significant protective associations utilised a circulating measure of lycopene(Reference Akbaraly, Faure and Gourlet22, Reference Polidori, Praticόc and Mangialasche23, Reference Min and Min29). Given the dietary factors influencing carotenoid bioavailability, it has been proposed that a relative carotenoid bioavailability measure be used to compare circulating levels of single carotenoids induced by fruit and vegetable intake with that of purified carotenoid supplementation(Reference van het Hof, West and Weststrate33). Such a measure would strengthen experimental research aimed at understanding the matrix-mediated effect on lycopene bioavailability and its subsequent relationship with cognition. Nevertheless, elucidating the health benefit of individual carotenoids is fraught with challenges given the common dietary sources and highly correlated nature of their presence in food. Thus, it cannot be ruled out that carotenoids may act synergistically as evidenced by the significant positive association reported by Kesse-Guyot et al.(Reference Kesse-Guyot, Andreeva and Ducros25) between total plasma carotenoids rather than lycopene alone and cognitive function. Such synergy between lycopene and other compounds in the food matrix has been highlighted by Burton-Freeman & Sesso(34) in a review evaluating the evidence on tomato intake v. lycopene supplementation on cardiovascular risk factors. In short, tomato supplementation elicited more favourable results on oxidative stress, inflammation, endothelial function and lipid metabolism whereas lycopene supplementation was more effective in blood pressure management. Taken collectively, traditional reductive approaches may not fully capture the synergy between foods and nutrients, specifically lycopene and other carotenoid compounds(Reference Hoffman, Schulze and Schienkiewitz35).
Age-related changes in metabolic processes as well as accumulation of ROS-induced damaged biomolecules with advancing age may influence overall lycopene status. For example, results from the Women's Health and Aging Study reveal that older adults have significantly lower circulating lycopene levels compared with younger individuals of matched ethnic and dietary history(Reference Semba, Patel and Ferrucci36). Factors influencing these findings include decreases in intestinal absorption of carotenoids with ageing and lifespan accumulation of health conditions associated with systemic oxidative stress(Reference Akhtar, Ahmed and Randhawa37, Reference Petyaev38). Additional determinants of circulating lycopene include age, lipid levels and smoking status(Reference Re, Mishra and Thane39, Reference Mayne, Cartmel and Silva40). Among studies included in the present review, six of the ten studies reported adjusting statistical models for at least one of these potential covariates(Reference Feart, Letenneur and Helmer14, Reference Akbaraly, Faure and Gourlet22–Reference Kesse-Guyot, Andreeva and Ducros25, Reference Min and Min29).
Furthermore, it must be acknowledged that decreases in both cognitive function and overall intake of carotenoids occur with advancing age(Reference Lauretani, Semba and Dayhoff-Brannigan41). More specifically, diet quality, whether it be due to physiological, psychological or socio-economic reasons, often declines with age(Reference Porter Starr, McDonald and Bales42). For example, a recent study examining dietary patterns among a diverse group of 416 men and women aged 65 years and older reported that fewer than 50 % of participants were meeting nutrient recommendations, with many particularly lacking optimal intake of fruits and vegetables(Reference Hsiao, Mitchell and Coffman43). In collective assessment of these potential mediating variables, age-related oxidative imbalances and low lycopene bioavailability along with diminished carotenoid intake represent the perfect storm for precipitating cognitive decline.
The present review reveals several important gaps in the literature related to lycopene intake and cognition. Divergent findings are probably attributed to the various study designs and cognitive measures employed; furthermore, the studies controlled for different covariates, examined different populations and utilised different outcome measures to evaluate cognitive performance (n 4), development of dementia (n 2) and influence on pre-existing dementia (n 4). Implications of the present review support the International Academy on Nutrition and Aging call for prospective studies of durational periods to sufficiently monitor diet and disease or cognitive decline(Reference Gillette Guyonnet, Abellan Van Kan and Andrieu44). Future research would also be strengthened by utilising a select battery of tests to allow for cross-study comparisons; additionally, more sensitive tests that tease apart different domains of cognition such as executive functioning, reasoning and short-term and working memory should be used rather than tests assessing global cognition only. Likewise, all future studies must control for known confounders in order to tease apart direct effects for guiding evidence-based practice. While this study provides implications for future research, it is not exempt from some limitations, namely the lack of meta-analytic assessment. Such analysis was not conducted due to incongruences in outcome measures coupled with the small number of articles in each outcome grouping which reduces statistical power for analysis. Also, database search filters used in identifying potential articles for inclusion in the present review were limited to articles with titles and abstracts available in English.
As low circulating lycopene is a predictor of all-cause mortality(Reference Shardell, Alley and Hicks45), this carotenoid compound warrants further investigation into its relationship with disease-specific mortality including dementia. At this time, there is insufficient evidence and a paucity of data to draw firm conclusions and to tease apart direct effects of lycopene alone, thus warranting additional research. Until such research is conducted, adopting a Mediterranean-type diet pattern may benefit cognitive longevity as greater adherence results in higher circulating concentrations of carotenoids including lycopene(Reference Daviglus, Plassman and Pirzada46).
Acknowledgements
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
K. M. C.-W., T. A. P. and A. C. E. developed the research question. All authors independently analysed and critically appraised articles included in the review. K. M. C.-W. and T. A. P. conducted the literature search and wrote the manuscript. A. C. E. edited the manuscript.
There were no conflicts of interest.