Many aspects of mammalian metabolism exhibit daily rhythms, driven by an integrated network of circadian clocks throughout the body(Reference Bass1). One consequence of metabolic rhythms is the interaction between time of day and food intake (‘chrononutrition’)(Reference Johnston, Ordovas and Scheer2). An emerging area of chrononutrition is time-restricted feeding (TRF), in which the daily duration of food intake is shortened(Reference Longo and Panda3). TRF reduces animals' body weight and improves markers of metabolic health without altered energy consumption(Reference Hatori, Vollmers and Zarrinpar4, Reference Sherman, Genzer and Cohen5). Beneficial effects of TRF schedules on murine body weight can occur within 8 weeks; lower fat mass and serum cholesterol, together with improved glucose tolerance, occur by the end of a 9-week protocol using a daily 15 h window of food availability(Reference Chaix, Zarrinpar and Miu6).
In humans 24 h rhythms of glucose homeostasis and postprandial responses are well known(Reference Van Cauter, Polonsky and Scheen7–Reference Johnston9), but TRF data are extremely limited. Most human TRF-related studies are limited by short study duration, use of extreme temporal restriction that is unrealistic for a long-term intervention, or change to nocturnal energy intake as occurs during Ramadan(Reference Rothschild, Hoddy and Jambazian10, Reference Antoni, Johnston and Collins11). There is a clear need to develop human TRF research, using protocols that reflect realistic interventions for free-living individuals. The present 10-week study therefore aimed to assess the feasibility of a TRF protocol in reducing the food intake window, in addition to the effect and variability in changes in several secondary outcomes (attrition rates, changes in dietary intake, body weight, adiposity and fasting cardiometabolic risk markers). Due to the lack of comparable TRF experiments in human subjects, this work was conducted in the first instance as a pilot study using a controlled study design comparing control v. treatment groups.
Methods
Participants
Sixteen healthy participants (BMI 20–39 kg/m2) aged 29–57 years were recruited. No a priori sample size was selected with the intention that data obtained from this pilot study would be used to inform power calculations for future work. Participants were weight-stable (±2 kg) over the preceding 6 months and had no significant medical history. Participants were excluded if they had travelled across more than two time zones within the month preceding the study or if they had participated in rotating or night shift work for more than 6 months. The study received a favourable ethical opinion from the University of Surrey Ethics Committee. Written, informed consent was obtained from all participants.
Study design
The study protocol is provided in Fig. 1(a). All participants undertook a 2-week baseline period, during which they followed habitual sleep–wake and feed–fast cycles. The timing and composition of energy intake were recorded in diet diaries over the final 4 d. At the end of the baseline period, participants made an initial morning laboratory visit. Participants abstained from alcohol and strenuous exercise for 24 h before the visit, consumed their preceding evening meal before 20.00 hours and therefore arrived following at least a 12 h fasting period. Body weight and composition were measured by bioimpedance (Tanita MC180A; Tanita Corp.). A fasting venous blood sample was taken into K-EDTA tubes (TAG, cholesterol, insulin analysis) and sodium oxalate tubes (glucose analysis).
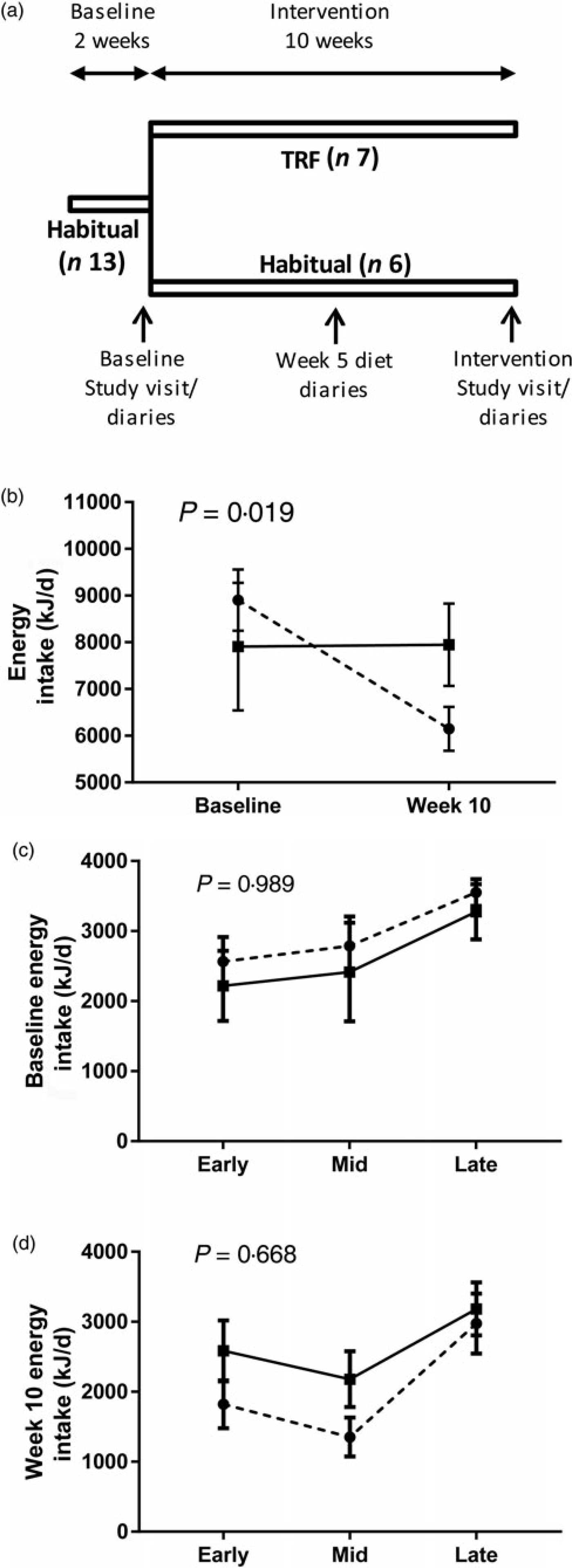
Fig. 1. Study design and effect of time-restricted feeding (TRF) on food intake. (a) Participants had 2-week baseline on habitual meal times and were then split into one of two groups for a 10-week intervention period; a control group maintained habitual meal times, whereas a TRF group restricted their daily feeding duration by 3 h. Diet, body weight, adiposity and fasting blood markers were assessed in the final week of the baseline and intervention periods. Dietary assessment was also made during week 5 of the intervention period. (b) Average daily energy intake in both groups at baseline and 10 weeks of the interventions. (c) and (d) Distribution of daily energy intake in each group during assessments at (c) baseline and (d) week 10 of the intervention period. Data are presented as means, with standard errors represented by vertical bars. –––, Control group (n 5); ---, TRF group (n 7). P values represent the group x time interactions.
After the initial laboratory visit, participants were assigned to the control (n 7) or TRF (n 9) group, ensuring no statistical differences in average age, BMI and body fat between groups. Both groups undertook a 10-week intervention period at home. The TRF group delayed first energy intake of the day and advanced last energy intake of the day, each by 1·5 h, compared with their dietary patterns calculated from the baseline diaries. The symmetrical compression of energy intake in the TRF group was chosen to minimise possible effects of morning v. evening differences in metabolism and thus increase likelihood that physiological changes were due to feeding duration per se, rather than time-of-day effects. Control participants maintained the dietary patterns reported in their baseline diaries. Both groups were asked to maintain habitual sleep–wake patterns. The timing and composition of energy intake were recorded in diet diaries over four consecutive days on two occasions during the intervention: mid-way through the intervention period (week 5) and in the final week (week 10). At the end of the 10th intervention week, participants made a repeat laboratory visit and were asked to consume the same evening meal as they did prior to the first laboratory visit. Completing participants were also given a questionnaire to assess their subjective experience of following the dietary pattern (Table 1).
Table 1. Baseline characteristics for study completers in time-restricted feeding (TRF) and control groups
(Mean values with their standard errors; numbers of participants)
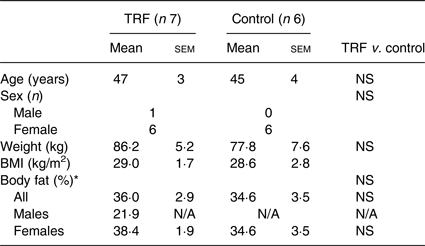
N/A, not applicable.
* Bioimpedance.
Dietary analysis
Participants recorded food intake in validated diet diaries(Reference McKeown, Day and Welch12), which included pictorial guides to aid portion size estimations when exact weights could not be provided. Diet diary analysis was performed with Diet Plan 7 (Forestfield Software), using the McCance and Widdowson's composition of foods integrated dataset. Generic foods in the nutritional analysis program were used unless specific food brands were provided, in which case nutritional composition information was manually inputted as a user added food. Recorded time of first and last energy-containing food was used to calculate the daily eating window. To assess for changes in daily energy intake distribution, each individual participant's daily eating window was divided into three time periods (thirds): early, mid, and late.
Analysis of fasting blood samples
Blood plasma aliquots were stored at −20°C. Insulin was analysed using ELISA (Millipore); glucose, TAG, total cholesterol and HDL-cholesterol were analysed using commercial kits for the ILAB650 (Instrumentation Laboratory). LDL-cholesterol was calculated using the Friedewald equation(Reference Friedewald, Levy and Fredrickson13). Intra-assay CV were <5 %.
Questionnaire
An exit questionnaire was devised for the study to allow participants to provide a subjective assessment of the intervention and to suggest modifications to the TRF protocol which could be used by future studies to improve compliance. Questions included: (1) ‘How difficult did you find the intervention?’; (2) ‘Do you think you could maintain this protocol for longer than 10 weeks?’; (3) ‘What were your main reasons for non-compliance?’; (4) ‘Do you think the plan made you eat differently?’; (5) ‘Do you think you might carry on with the plan in any form?’.
Statistics
Statistical analyses were performed on data from participants who completed the study. Data were tested for normality using the Shapiro–Wilk test. Differences between intervention groups at baseline were assessed using independent t tests for continuous variables or the χ2 test for categorical variables, with any significant differences reported in the text. Paired t tests were used to assess for within-group changes over the intervention period. Other data were analysed using a two-way ANOVA, with the period of daily eating window or laboratory visit as a repeated measure. Where data were not normally distributed, non-parametric tests were used to assess between-group differences (Mann–Whitney U) and within-group changes (Wilcoxon signed-rank test). Moreover, due to the small sample size, data were also inspected for outliers; where outliers were observed (adiposity) but exhibited normality using the Shapiro–Wilk test, non-parametric tests were also used owing to their greater resilience against outliers. Due to lower completion rates, intervention week 5 dietary intake data are presented as Supplementary material (Supplementary Fig. S1 and Table S2), but are not included in the primary statistical analyses. We tested the hypothesis that the TRF intervention would result in a significant group × time interaction. Data are presented as mean values with their standard errors unless otherwise stated.
Results and discussion
Recruitment, attrition and feasibility of time-restricted feeding intervention
A total of sixteen healthy and overweight individuals were initially recruited into the study. Overall, attrition rates were low. One control subject dropped out due to faintness during blood collection at the first clinical visit so did not commence the study. Within the TRF group, seven of the nine participants successfully completed the 10-week intervention. One TRF participant was lost to follow-up and so the reason for drop out is unknown but may relate to the TRF intervention. The second TRF participant was excluded as they had participated in another research project (resulting in changes to their habitual diet), and therefore the reason for drop out was not due to difficulties with adhering to the TRF intervention.
Participants in the TRF group were asked to delay and advance the timing of their first and last energy intakes, respectively, by 1·5 h, with no restrictions placed on meal frequency or overall energy intake. This differs from recent studies in which male participants ate three prescribed meals per d(Reference Moro, Tinsley and Bianco14, Reference Sutton, Beyl and Early15). Moreover, the symmetrical change in feeding duration differs from asymmetrical reductions in feeding duration in which physiological changes could result from time-of-day effects in addition to any consequence of TRF. In our study, the total eating window was reduced by an average of about 4·5 h (from 743 (sem 32) and 517 (sem 22) min/d) based on comparisons between 4 d dietary records kept at baseline and the final week of the intervention; participants achieved their target eating window (≥3 h reduction v. baseline) on average about 2·5/4 d in week 10. By comparison, there was minimal change in the feeding window of the control group (652 (sem 50) to 677 (sem 41) min/d), resulting in a significant group × time interaction (P < 0·001; two-way repeated-measures ANOVA).
Therefore, these data suggest that the TRF intervention was achievable over a 10-week time-frame. However, many participants found it difficult to stick to the regimen every day, and questionnaire data revealed an average difficulty score of 7/10 (1: easy; 10: extremely difficult) among TRF participants. Some common themes emerged from the questionnaires, with most participants reporting TRF protocol deviations occurring due to social eating/drinking events. Other reasons reported by one participant included illness and a late running work meeting. Of TRF participants, 57 % (n 4) felt they could not have maintained the TRF protocol beyond 10 weeks. This was mainly due to incompatibility with family/social life, with one participant noting that they found sticking to the TRF regimen relatively easy as they lived alone. Of the participants, 43 % (n 3) felt they would consider continuing the protocol if it had demonstrable health benefits or would consider continuing a more flexible protocol, e.g. Monday–Friday, earlier dinners or later breakfasts/dinners. An important consideration for future work is therefore the positioning of the TRF window within the 24 h day, and whether the TRF window should remain constant or can vary.
Dietary intake
Consistent with other human studies(Reference Gill and Panda16–Reference Tinsley, Forsse and Butler18), the TRF intervention also caused a decrease in daily energy intake despite ad libitum food access, indicated by a significant group × time interaction (Fig. 1(b)). This was corroborated by questionnaire responses, with 57 % (n 4) of participants noting a reduction in food intake either due to reduced appetite, reduced duration of eating opportunities and/or reduced snacking (particularly in the evening). Overall, daily energy intake distribution was unaffected, as there was no significant group × time interaction at baseline (Fig. 1(c)) or intervention week 10 (Fig. 1(d)). Some TRF participants reported eating ‘less healthily’ via increased consumption of convenience foods due to time restrictions with food preparation (n 3), whilst others consumed less alcohol (n 5), presumably due to reduced evening social opportunities, although this did not translate into significant between-group differences in the changes in macronutrient intakes (Supplementary Table S1). Analysis of diet diaries from participants who completed them at baseline, intervention week 5 and intervention week 10 indicates that changes in dietary intake were similar throughout the intervention period (Supplementary Fig. S1 and Table S2).
Body weight
Despite the observed changes in energy intake, body weight was not significantly changed after either the control (from 77·8 (sem 7·5) to 77·3 (sem 7·7) kg; P = 0·114) or TRF (from 86·2 (sem 5·2) to 85·5 (sem 5·2) kg; P = 0·374) interventions, and this was comparable between groups (P = 0·748; two-way repeated-measures ANOVA). Similarly, there was no significant interaction between assessment group × time for BMI (P = 0·788; two-way repeated-measures ANOVA). The control group had BMI of 28·6 (sem 2·8) and 28·4 (sem 2·9) kg/m2, whereas the TRF group had BMI of 29·0 (sem 1·7) and 28·7 (sem 1·8) kg/m2 (baseline v. post-intervention, respectively).
Adiposity
In contrast to the body weight data, there was a significant (P = 0·047; Mann–Whitney U test) effect of dietary intervention on percentage body fat (Fig. 2(a)). Indeed, all members of the TRF group exhibited lower body fat by the end of the intervention period (Fig. 2(c)), with an average reduction of 1·9 (sem 0·3) percentage points after TRF. Lean body mass was not measured, and this may explain the discrepancy in the body weight data, whereby net body weight remained unchanged despite reductions in adiposity and food intake. Despite this, the consistency of the observed reduction in adiposity among all TRF participants suggests that these data represent a true treatment effect; nonetheless, replication is required given the type 1 error risk.
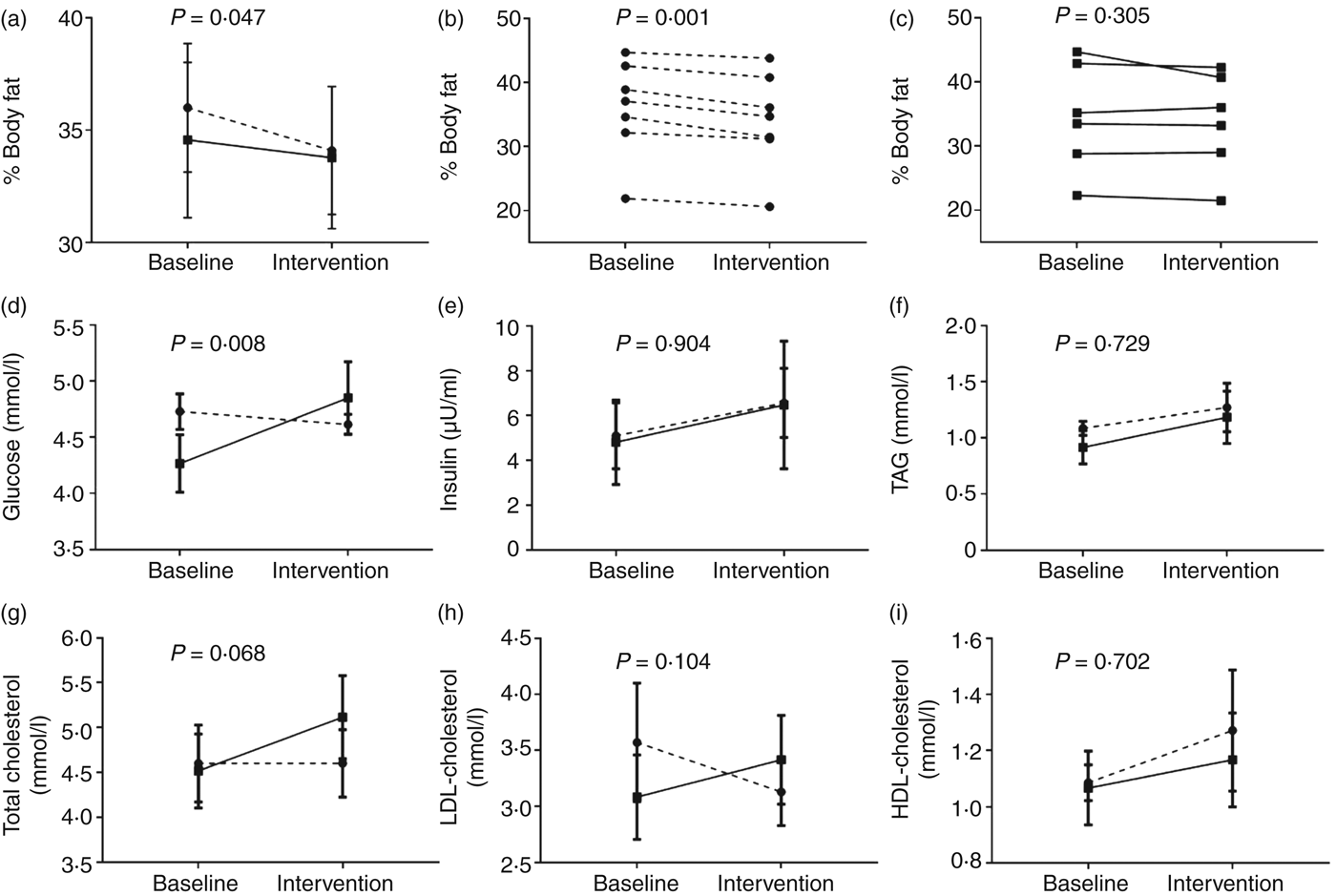
Fig. 2. Effect of time-restricted feeding (TRF) on body fat and fasting plasma markers. After a 2-week baseline period, participants maintained habitual feeding patterns or restricted their daily feeding duration by 3 h for 10 weeks, with data collected at the end of the baseline and 10-week intervention periods. (a) Average percentage body fat in both groups at the end of the baseline and intervention periods. (b) and (c) Percentage body fat in each individual in the (b) TRF and (c) control groups at the end of the baseline and intervention periods. (d)–(i) Fasting plasma markers in both groups at the end of the baseline and intervention periods. –––, Control group (n 6); ---, TRF group (n 7). In panels (b) and (c), data are individual values, P values are within-group changes; in other panels, data are means, with standard errors represented by vertical bars. P values represent the group x time interactions.
One purported mechanism for the health benefits of TRF is that a higher percentage of energy is consumed during a restricted phase of the endogenous circadian cycle. Additionally, TRF may exert benefits by increasing the length of the daily fast(Reference Longo and Mattson19). However, given our participants consumed significantly less energy per d as a result of a TRF intervention (despite ad libitum food access), this is likely to be a key driver of the observed changes in adiposity(Reference Gill and Panda16–Reference Tinsley, Forsse and Butler18).
Fasting plasma biochemistry
Although there was no significant difference in fasting plasma glucose between the two groups during baseline conditions, a significant diet × group interaction was observed for the change in fasting plasma glucose (Fig. 2(d)). This was largely driven by elevated concentrations consistently observed among all participants in the control group at the end of the intervention period but the reason for this change is unknown; given there were no reported significant changes in dietary intake in the control group, this is suggestive of changes in other unknown factor(s). There were modest changes in other metabolic disease risk markers, including trends in favour of a reduction in LDL-cholesterol; however these were not significantly different from the control group (Fig. 2(e)–(i)). This is perhaps unsurprising given the small, healthy cohort studied. Nonetheless, data provide valuable pilot information that can be used to design appropriately powered future studies that include assessment of metabolic physiology.
Strengths, weaknesses and impact on future experimental design
The particular strengths of the present study include its controlled study design and modest but achievable TRF protocol. By contrast, many published TRF-related studies lasted for a maximum of 4 weeks, involved extreme temporal restriction, or included alternate TRF/non-TRF days; others are observational Ramadan studies in which participants changed not only their feeding duration but timing from a diurnal to nocturnal feeding pattern(Reference Rothschild, Hoddy and Jambazian10, Reference Antoni, Johnston and Collins11). Our exit questionnaire also provides insights into the factors affecting the acceptability of TRF and how this could be improved, for instance by reducing the number of TRF days per week. TRF for 5 d per week has proved efficacious in rodents in terms of reducing adiposity and improving metabolic markers(Reference Chaix, Zarrinpar and Miu6) but the efficacy in humans remains to be established.
This pilot study did have limitations. The study was conducted in a small group of both healthy and overweight well-motivated, predominantly female, participants within a high-income region in Surrey recruited as part of a television documentary. This limits generalisability of the findings to other population groups and increases the risk of type 1 and 2 errors. Other limitations include the reliance upon self-reported energy intakes and the use of bioimpedance to assess changes in body composition. Whilst bioimpedance is validated against more robust anthropometrical techniques such as dual-energy X-ray absorptiometry, it does systematically underestimate body fat with increasing adiposity and can be influenced by hydration and hormonal status(Reference Frisard, Greenway and Delany20). Lastly, whilst participants were asked to maintain habitual activity levels, no formal monitoring of physical activity was conducted. It is therefore unknown whether TRF led to compensatory changes in physical activity, which would also influence overall energy balance. Although assessment of metabolic markers revealed non-significant changes in plasma TAG/cholesterol concentration, our data provide valuable insight into the magnitude and variation of expected responses, which will inform experimental design and power calculations for future experiments.
Conclusion
In conclusion, data from this 10-week pilot study provide initial evidence that a modest contraction of the eating window is achievable within a free-living human population. Moreover, the TRF intervention elicited favourable changes in dietary intakes, accompanied by a reduction in adiposity. The importance of this ‘unintentional’ dietary modification is important in the context of our obesogenic environment. However, participation in the study did affect social eating/drinking opportunities in the evening. Larger studies are now required and, based on our preliminary findings, should also carefully consider personal/social considerations of participants undertaking TRF protocols to maximise compliance.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/jns.2018.13
Acknowledgements
The authors thank Leila Finikarides for recruitment and help with study management and Dr Jo Sier for analysis of plasma samples.
Financial support was received from the University of Surrey and the British Broadcasting Corporation.
All authors conducted the experiment. R. A. analysed data and wrote the manuscript; T. M. R. and M. D. R. revised the manuscript; J. D. J. analysed data and wrote the manuscript.
J. D. J. has performed consultancy work for Kellogg Marketing and Sales Company (UK) Limited, and collaborates with the Nestlé Institute of Health Sciences.




