Introduction
Cephalopod mollusks have been a prominent component of marine ecosystems for the past half billion years, and fossils of their mineralized shells provide an often detailed chronicle of their later evolutionary history.
Cephalopoda is divided into two major lineages. The fossil record of nautiloids begins in the latest Cambrian, proliferates through the Ordovician, and dwindles towards the present day. The neocoleoid lineage is most familiar to paleontologists through its stem-group representatives, namely the ammonoids and belemnoids, which are abundant from the Devonian until their end-Cretaceous extinction (House, Reference House1985; Teichert, Reference Teichert1986; Holland, Reference Holland1987; Kröger et al., Reference Kröger, Vinther and Fuchs2011); extant Neocoleoidea exhibit diminutive, non-mineralized or chemically fragile shells, and thus require unusual preservational conditions in order to enter the fossil record (Kear et al., Reference Kear, Briggs and Donovan1995).
Whereas exceptional Mesozoic specimens greatly illuminate the diversification of neocoleoids (Doguzhaeva et al., Reference Doguzhaeva, Summesberger, Mutvei and Brandstaetter2007; Yancey et al., Reference Yancey, Garvie and Wicksten2010), earlier taxa are often difficult to place phylogenetically (Sutton et al., Reference Sutton, Perales-Raya and Gilbert2016), a problem compounded by the increasing scarcity of exceptional preservation as one goes deeper into the Paleozoic. With almost no non-biomineralized cephalopod tissue known prior to the Carboniferous Period (Klug and Lehmann, Reference Klug, Lehmann, Klug, Korn, De Baets, Kruta and Mapes2015), there is little direct fossil evidence—ammonoids and belemnoids notwithstanding—from which to reconstruct the earliest emergence of the coleoids.
The oldest uncontroversial cephalopods are late Cambrian phragmocones—chambered shells in which adjacent chambers are connected by a siphuncular tube, which represents a cephalopod synapomorphy. The consensus view is that a shell was inherited from a molluscan common ancestor, with several chambered ‘monoplacophoran’ taxa (Yochelson et al., Reference Yochelson, Flower and Webers1973; Brock and Paterson, Reference Brock and Paterson2004) representing candidate intermediate forms. On this view, weakly mineralized skeletal apparatuses such as the coleoid gladius, pen, or pro-ostracum arose through the reduction of a robust mineralized shell, perhaps on multiple occasions, but no earlier than the Carboniferous (Kröger et al., Reference Kröger, Vinther and Fuchs2011; Doguzhaeva and Mapes, Reference Doguzhaeva and Mapes2015).
This model has no place for the problematic Nectocaris pteryx Conway Morris, Reference Conway Morris1976, a non-mineralizing early Cambrian organism from Burgess Shale-type deposits that strikingly resembles modern coleoids (Smith and Caron, Reference Smith and Caron2010; Smith, Reference Smith2013). To some extent, this similarity reflects characteristics that may have arisen convergently: camera-type eyes, lateral fins, denticulate mouthparts, and anterior tentacles may each have arisen more than once among Metazoa (Mazurek and Zatoń, Reference Mazurek and Zatoń2011). Insofar as unique combinations of individually non-unique characteristics can be instructive (Butterfield, Reference Butterfield2005), it is noteworthy that cephalopods are the only organisms to display this particular combination. But a more definitive characteristic (Runnegar, Reference Runnegar2011) is a wide axial cavity that contains a pair of gills and opens through a ventrally directed anterior funnel. If this is correctly interpreted as a cephalopod mantle cavity (Smith and Caron, Reference Smith and Caron2010; Smith, Reference Smith2013), then it represents a cephalopod synapomorphy, and ascribes Nectocaris to the cephalopods as surely as a siphunculate phragmocone would.
The suggestion that this void might instead represent a gut (Kröger et al., Reference Kröger, Vinther and Fuchs2011; Runnegar, Reference Runnegar2011), which is presumably the basis for reconstructing a straight gut in Nectocaris (Kröger et al., Reference Kröger, Vinther and Fuchs2011; Klug et al., Reference Klug, Kröger, Vinther, Fuchs, Klug, Korn, De Baets, Kruta and Mapes2015), has been firmly discounted (Smith, Reference Smith2013). No gut has ever been observed in Nectocaris (Smith and Caron, Reference Smith and Caron2011), though the anterior location of the funnel implies that the gut, along with the body axis, was folded into a U-shape during development (Runnegar, Reference Runnegar2011).
Taken together, Nectocaris presents two characters known only in Cephalopoda—an axial mantle cavity and anterior funnel—along with a suite of characters that are only found together in cephalopods: internal gills, camera-type eyes, flexible muscular tentacles, muscular lateral fins with criss-crossing connective tissue, and denticulate chevron-shaped mouthparts.
Of course, no list of synapomorphies can conclusively establish affinity, and it remains possible that Nectocaris embodies extreme evolutionary convergence from an undetermined metazoan (or indeed non-metazoan) lineage (Kröger et al., Reference Kröger, Vinther and Fuchs2011; Mazurek and Zatoń, Reference Mazurek and Zatoń2011; Runnegar, Reference Runnegar2011). Even so, it is difficult to pinpoint a lineage from which a nectocaridid-like morphology might plausibly be derived. There is no clear indication of an ecdysozoan, deuterostome, or gnathiferan affinity, and those trochophore phyla with complex free-living body plans have a reasonably well-constrained evolutionary history: mollusks, annelids, and brachiopods seem to have evolved from a grade of creeping organisms with dorsal imbricating scleritomes (Skovsted et al., Reference Skovsted, Betts, Topper and Brock2015; Zhang et al., Reference Zhang, Smith and Shu2015; Sun et al., Reference Sun, Smith, Zeng, Zhao, Li and Zhu2018) that bear no obvious similarity to Nectocaris.
To further inform the evolutionary position of Nectocaris, I here describe a new Katian (Late Ordovician) nectocaridid with an internal, non-mineralized skeletal element. Nectocotis rusmithi n. gen. n. sp. demonstrates that nectocaridids survived the terminal Cambrian extinction event that decimated phragmocone-bearing cephalopods (Kröger, Reference Kröger2013), and hints that coleoids, rather than nautiloids, are the most appropriate model for the ancestral cephalopod.
Materials and methods
This study concerns the part and partial counterpart of a single specimen from the Katian (Upper Ordovician, ~450 Ma) Whetstone Gulf Formation, Lorraine Group, Lewis County, New York State. The specimen, which measures 11 mm from apex to anterior margin of funnel and 5 mm at point of maximum width (Fig. 1; Smith, Reference Smith2019), occurs in a massive, dark gray siltstone that contains rare sub-mm pyrite crystals. In contrast to the pyritization for which the Whetstone Gulf Formation is known (Farrell et al., Reference Farrell, Martin, Hagadorn, Whiteley and Briggs2009), this specimen is preserved in Burgess Shale fashion (Butterfield et al., Reference Butterfield, Balthasar and Wilson2007). Blue coloration under bright-field illumination denotes the presence of aluminosilicate minerals that presumably templated an original carbon film. As with Burgess Shale fossils, these films appear dark under cross-polarized light (Fig. 1.1), but are brighter, becoming difficult to distinguish from the matrix, under non-polarized dark-field illumination (Fig. 1.2, 1.3).
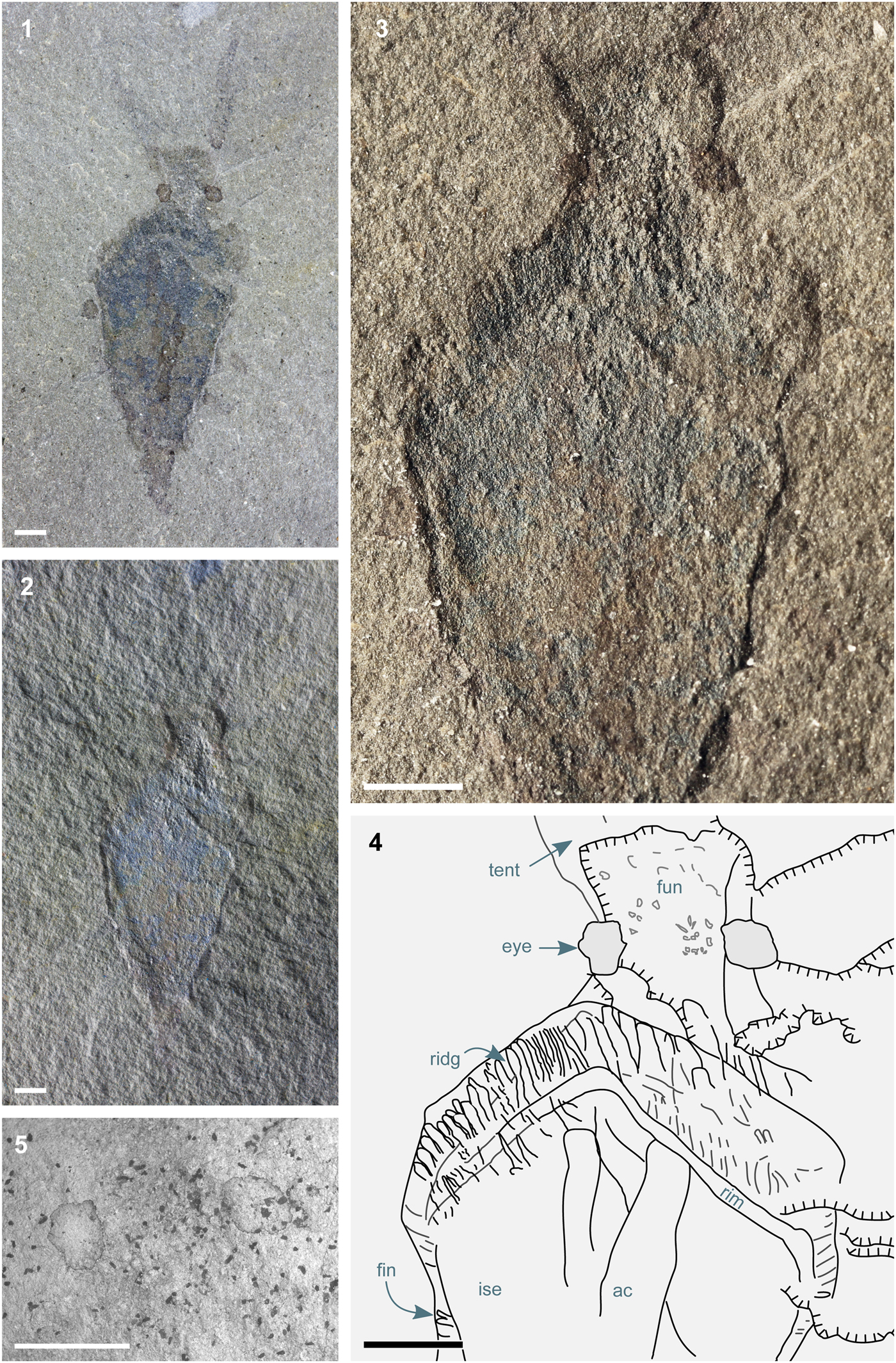
Figure 1. Nectocotis rusmithi n. gen. n. sp. (ROM IP 65341). (1) Dorsal surface of complete specimen, bright-field illumination, crossed polars; (2) dark-field illumination, relief emphasized using the Grain Extract algorithm (GNU Image Manipulation Program 2.10.10, www.gimp.org) to superimpose images taken under two opposite illumination directions; pixels assigned color values from bright-field image; (3) dark-field illumination; (4) sketch summarizing features visible under different lighting conditions; (5) scanning electron micrograph of head region, showing relief and distinct composition of eyes. High-resolution images are available at FigShare (Smith, Reference Smith2019). All scale bars represent 1 mm. Abbreviations: ac, decayed contents of axial cavity (gills?); fun, funnel; fin, fin; ise, internal skeletal element; ridg, ridges in fin; rim, rim of kite-shaped structure; tent, tentacle.
Repository and institutional abbreviation
Material is accessioned at the Royal Ontario Museum (ROM), Toronto, Canada.
Systematic paleontology
Family Nectocarididae Conway Morris Reference Conway Morris1976
Genus Nectocotis new genus
Type species
Nectocotis rusmithi new species, by monotypy.
Diagnosis
As for type species, by monotypy.
Etymology
Reflecting the origin of the material from the Whetstone (Latin cotis) Gulf Formation.
Remarks
The key difference between Nectocotis n. gen. and Nectocaris is the presence of a robust internal skeletal component within the dorsal body region.
Nectocotis rusmithi new species
Figure 1
Holotype and only known specimen
ROM IP 65341.
Diagnosis
Nectocaridid whose body is spanned by a robust field in the shape of a convex Euclidian kite.
Description
The overall construction of the specimen closely resembles Nectocaris pteryx (Smith and Caron, Reference Smith and Caron2010; Smith, Reference Smith2013). The body measures 4.4 mm at its widest point and 10.0 mm in length, discounting the head. Its widest point is 7 mm from the posterior. A gently flaring ventral structure extends 1.9 mm from the anteriormost part of the body, increasing in width from 1.2 mm to 2.0 mm at its distal end; this corresponds in position and shape to the Nectocaris funnel, while being proportionally larger in relation to the body (as fluid dynamic considerations would predict of an exhalent siphon at small body size; Smith, Reference Smith2013). A pair of prominent eyes are preserved as dark structures with a diagenetic infill (Fig. 1.1, 1.5), presumably denoting a high concentration of preserved carbon, as in Nectocaris. A pair of smooth-margined tentacles (of which the basal 3.5 mm is preserved) emerge anterodorsally from the head. Dark axial elements in the body region (‘ac’ in Fig. 1) presumably represent gills within an axial cavity, but lack the preservational fidelity necessary for a confident interpretation.
The dorsal body region of Nectocotis rusmithi n. gen. n. sp. is predominantly occupied by a flat structure that I interpret as an internal skeletal element. Its central region is flatter than the uneven fracture surface of the surrounding matrix, whereas its margins exhibit prominent relief (Fig. 1.2, 1.3); taken together, these observations denote a structure that was originally robust and inflexible enough to resist deformation and compression. This resilience cannot represent early permineralization of muscular tissue: the muscular tentacles and funnel are preserved without relief, as in equivalent specimens in the Burgess Shale (Smith and Caron, Reference Smith and Caron2010; Smith, Reference Smith2013). The element occupies almost the full width of the organism, in contrast with the medial axial cavity observed in Nectocaris. It is difficult to see how rapid mineralization of digestive tissue or gills could give rise to an entity with a well-defined quadrilateral margin. The only satisfactory account for the shape and relief of the structure is that it represents a robust (though seemingly not mineralized) skeletal element.
This skeletal element is laterally surrounded by a continuous region of soft tissue interpreted as a fin, based on its position and lateral deformation (cf. Nectocaris, Smith Reference Smith2013). Anterior to the skeletal element, the fins bear a series of 100 μm wide ridges (Fig. 1.3, Fig. 1.4) similar in proportion, orientation, and three-dimensionality to the coarse stripes in the fins of Nectocaris (Smith, Reference Smith2013). The fins overlap the skeletal element on the (ventrally preserved) fossil, whereas the tentacles, eyes, and head lie in a plane deeper in the rock, and thus dorsal to the skeletal element. Being sandwiched between these two layers of soft tissue and surrounded by the fins, the skeletal element is necessarily internal.
Etymology
Patronym, for R.D.A. Smith, who generously donated the specimen from his private collections.
Remarks
The presence of an internal skeletal element distinguishes Nectocotis n. gen. from Nectocaris. If such an element were present in Nectocaris during life, its absence in fossils would be hard to explain, given the routine association of rigid skeletal elements with relief in Burgess Shale-type deposits. The robust internal element in the posterior body of a single large specimen (Smith, Reference Smith2013, fig. 11A) is the only possible candidate, but because this feature is diminutive, differs in shape, and occurs in but a single specimen, its homology with the newly described skeletal element must be considered uncertain.
Discussion
An internal skeletal element represents a further addition to the list of cephalopodan features present in nectocaridids. One of the most fundamental principles of phylogenetic systematics is Hennig's auxiliary principle (Hennig, Reference Hennig1953), which states that similarities should be assumed to reflect kinship rather than convergence (De Laet, Reference De Laet and Albert2005; Mooi and Gill, Reference Mooi, Gill, Williams, Schmitt and Wheeler2016). Only by interpreting nectocaridids as total group cephalopods (Fig. 2) can cephalopod similarities (funnel, internal gills, jet propulsion, tentacles, prominent eyes) be attributed to common ancestry. (Features absent in nectocaridids—multiple arms; chitinous beak; shell chambers—likely arose later in the cephalopod lineage.)
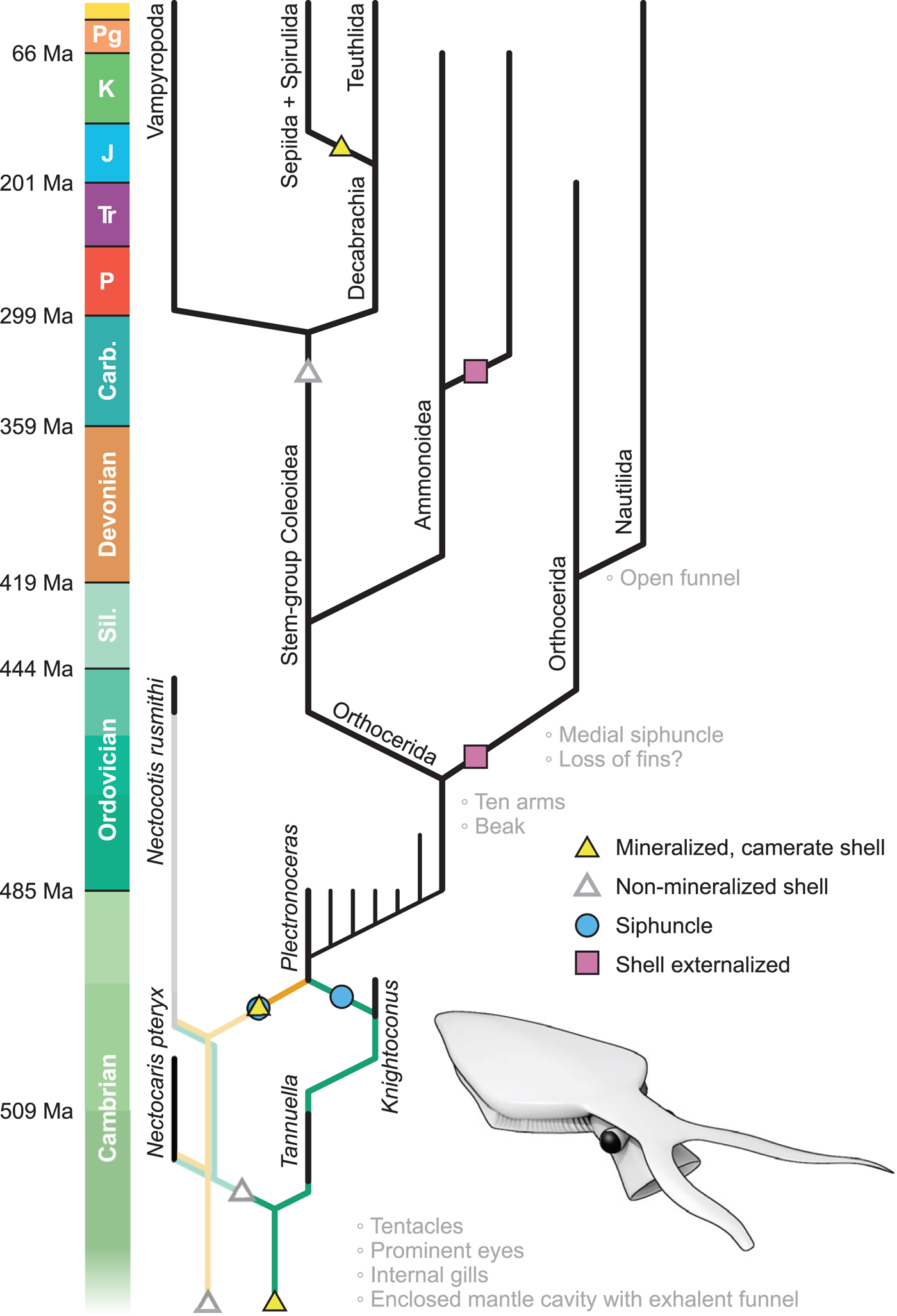
Figure 2. Simplified cephalopod phylogram. The absence of unambiguous shelly cephalopods in the early–mid Cambrian may be filled by the taxonomically ambiguous genera Tannuella (Brock and Paterson, Reference Brock and Paterson2004) and Knightoconus (Yochelson et al., Reference Yochelson, Flower and Webers1973) (dark green pathway, right), or may denote a primitively non-mineralized configuration (pale orange pathway, left). Bold lines indicate mineralized lineages; faint lines denote ghost lineages. Inferred origins of key apomorphies indicated; time plotted to logarithmic scale. Inset: reconstruction of Nectocotis rusmithi n. gen. n. sp.
If a mineralized shell were present in the ancestral cephalopod, then this position creates a 30 million year stratigraphic gap before the first undoubted cephalopod, the mineralized and siphunculate Plectronoceras. On a conservative view, this gap may require no special explanation: gaps of this magnitude do occur, for example, in the Cambrian shelly fossil record (e.g., Runnegar and Pojeta, Reference Runnegar and Pojeta1992) and in Mesozoic coleoids (see Brayard et al., Reference Brayard, Krumenacker, Botting, Jenks, Bylund, Fara, Vennin, Olivier, Goudemand, Saucède, Charbonnier, Romano, Doguzhaeva, Thuy, Hautmann, Stephen, Thomazo and Escarguel2017). More proactively, such a gap might be filled by camerate shelly fossils such as Knightoconus and Tannuella (Yochelson et al., Reference Yochelson, Flower and Webers1973; Brock and Paterson, Reference Brock and Paterson2004) (Fig. 2, dark green)—though there is no hard reason that these taxa must be cephalopods, as septa have evolved independently many times, including in lophophorates (the hyolith Cupitheca, Skovsted et al., Reference Skovsted, Pan, Topper, Betts, Li and Brock2016), gastropods (Fretter and Graham, Reference Fretter and Graham1978), tentaculitoids (Weedon, Reference Weedon1990), and foramanifera. Alternatively, this stratigraphic gap may indicate that the earliest cephalopod phragmocones, like the nectocaridid skeletal element, lacked biomineralization—in which case Plectronoceras represents the earliest cephalopod seen to mineralize its shell field (Fig. 2, orange). On this view, the shell field—a synapomorphy of Conchifera (Kniprath, Reference Kniprath1981; Hohagen and Jackson, Reference Hohagen and Jackson2013)—is a primitively non-mineralized organ, consistent with its lack of biomineralization early in ontogeny (Bandel, Reference Bandel and Carter1989; Checa et al., Reference Checa, Cartwright, Sánchez-Almazo, Andrade and Ruiz-Raya2015), and the non-mineralized nature of early mollusk relatives (Caron et al., Reference Caron, Scheltema, Schander and Rudkin2006). Parsimony analysis denotes that the mineralization of a non-mineralized shell field is not a unique event in cephalopod evolution, having occurred in Spirulida and conceivably Sepiida (Sutton et al., Reference Sutton, Perales-Raya and Gilbert2016).
The morphology of the earliest cephalopod fossils has traditionally been modeled on living Nautilus, but nectocaridids suggest that the ancestral cephalopod more closely resembled a coleoid—most significantly in bearing an internal shell field. There is no direct evidence (e.g., muscle scars) that the earliest cephalopod shells were external (Webers and Yochelson, Reference Webers and Yochelson1989). The recognition that a range of nautiloid, orthocerid, and ammonoid shells were internal (Turek and Manda, Reference Turek and Manda2012; Doguzhaeva and Mutvei, Reference Doguzhaeva, Mutvei, Klug, Korn, De Baets, Kruta and Mapes2015; Mutvei and Mapes, Reference Mutvei and Mapes2018) raises the possibility that shell externalization characterizes only a small subset of cephalopod lineages, including certain orthocerids (Gabbott, Reference Gabbott1999; Kröger et al., Reference Kröger, Servais and Zhang2009), modern nautiloids and the ectocochleate ammonoids (Maeda and Seilacher, Reference Maeda, Seilacher, Landman, Tanabe and Davis1996).
Whatever their exact phylogenetic placement, nectocaridids indicate that the earliest cephalopod-like organisms had a high specific biomass: a correlate of power density and metabolic activity (O'Dor and Webber, Reference O'Dor and Webber1991; Bambach, Reference Bambach1993; Brown et al., Reference Brown, Gillooly, Allen, Savage and West2004). In contrast, the high shell volume in Cambro-Ordovician nautiloids denotes a lower metabolic rate and a higher physiological efficiency (O'Dor et al., Reference O'Dor, Forsythe, Webber, Wells and Wells1993; Boutilier et al., Reference Boutilier, West, Pogson, Mesa, Wells and Wells1996). This metabolic trend mirrors that observed in the brachiopod total group through the Cambrian (Sun et al., Reference Sun, Smith, Zeng, Zhao, Li and Zhu2018), suggesting that early neocoleoid-like organisms such as nectocaridids were largely supplanted by metabolically conservative, externally shelled, passively buoyant nautiloids in response to declining oxygen and energy availability in the late Cambrian/Early Ordovician. Low-productivity Paleozoic oceans (Bambach, Reference Bambach1993) saw a burgeoning of nautiloids, whereas nectocaridids did not proliferate, despite persisting until at least the latest Ordovician: metabolically expensive jet propulsion was excluded from niches for fast, highly active swimmers until the advent of the Carboniferous.
Acknowledgments
I thank R.D.A. Smith for obtaining and generously donating the fossil material, and M. Yacobucci and anonymous referees for constructive reviews.
Accessibility of supplemental data
High resolution light and electron micrographs are available from FigShare at https://doi.org/10.6084/m9.figshare.c.3953191 (Smith, Reference Smith2019).



