Non-technical Summary
Extinct fossil mammals, herein referred to as the Maysville Local Fauna (L.F.)., are described from the Martin Marietta Belgrade Quarry in eastern North Carolina. The fossils have been collected over the past several decades by professional and citizen (amateur) paleontologists. These fossils come from a single sedimentary unit called the Belgrade Formation that has previously been dated as late Oligocene or Early Miocene age (about 17 to 21 million years ago).
At least a dozen fossil mammal taxa of the Maysville L.F. are described here from the Belgrade Formation. These include five placental orders such as beavers (Rodentia), lagomorphs, (Lagomorpha), carnivores (Carnivora, including mustelids and ailurids), odd-toed ungulates (Perissodactyla, including horses, rhinos, and tapirs), and even-toed ungulates (Artiodactyla, including peccaries, anthracotheres, entelodonts, and protoceratids). The stage of evolution of this mammal fauna indicates an Early Miocene age, most probably between about 21 and 17 million years ago.
The Maysville L.F. has similar land mammal species to those from localities elsewhere in North America, including Florida, Nebraska, and Texas. This was a very interesting time in land mammal evolution that has been termed the Runningwater Chronofauna, including the presence of several Old Word immigrant taxa (carnivores and rhino) that are index fossils to this time interval. Strontium ratios preserved in fossil shark teeth from the Belgrade Formation indicate an Early Miocene age of 21.4 ± 0.13 million years ago, similar to the land mammal age determinations. In contrast, analyses of bivalves (oyster and clams), presumably from another subunit within the same formation, indicate an older (28.0 ± 0.22 Ma and 27.6 ± 0.26 Ma) Late Oligocene age.
Introduction
The Early Miocene is of great importance to understand the macroevolution and distribution of land mammals in North America. It marks the origins and major adaptive radiations of many modern families of mammals, and considerable faunal dispersal coinciding with global sea-level changes (Webb and Opdyke, Reference Webb and Opdyke1995; Qiu, Reference Qiu2003; Tedford et al., Reference Tedford, Albright, Barnosky, Ferrusquia-Villafranca, Hunt, Storer, Swisher, Voorhies, Webb, Whistler and Woodburne2004). Here, we concentrate on the early part of the Miocene (ca. 23–16 Ma), which also encompasses the Aquitanian and Burdigalian marine stages (ICS, 2023). In terms of the North American Land Mammal Age (NALMA) biochronology, this same interval encompasses the late Arikareean through Hemingfordian biochrons (Tedford et al., Reference Tedford, Albright, Barnosky, Ferrusquia-Villafranca, Hunt, Storer, Swisher, Voorhies, Webb, Whistler and Woodburne2004). The latter will become of greater importance as we describe the biochronology of the land mammals discovered at the Martin Marietta Belgrade Quarry (MMBQ) in eastern North Carolina.
Early Miocene land mammal faunas are relatively abundant in the western part of North America at mid-latitudes, Florida, and Panama (Tedford et al., Reference Tedford, Albright, Barnosky, Ferrusquia-Villafranca, Hunt, Storer, Swisher, Voorhies, Webb, Whistler and Woodburne2004). In contrast, land mammal faunas of this age are rare in eastern North America, north of Florida. As such, the recovery of a small but biochronologically diagnostic assemblage of land mammals from MMBQ makes it an important contribution to understand Early Miocene biogeography. In addition, the Maysville Local Fauna (L.F.; Tedford, Reference Tedford1970) described here from the Belgrade Formation at MMBQ is of added importance because of interbedded terrestrial and marine faunas, which are likewise rare during this time in North America. The purpose of this contribution, therefore, is to describe the land mammals from MMBQ and to discuss their evolutionary, biochronological, and geographic significance.
Geological setting
The MMBQ (MSHA ID: 3100064) is located south of State Road 58, ~4 km southeast of the town of Maysville in Jones County in the Atlantic Coastal Plain region of eastern North Carolina. This open-pit mine is generally very fossiliferous, consisting primarily of marine invertebrates and sharks. The exact locations that produce fossils have changed over the years as mining operations have shifted laterally.
An Upper Cretaceous and Cenozoic stratigraphic succession is generally exposed in this region at numerous localities (Ward et al., Reference Ward, Lawrence and Blackwelder1978). At MMBQ, a ~8-m-thick section (section 20 in Ward et al., Reference Ward, Lawrence and Blackwelder1978) includes, in stratigraphic sequence, the Oligocene River Bend Formation, Lower Miocene Belgrade Formation, Pliocene Duplin and Croatan formations, and unstudied Pleistocene unconsolidated deposits at the top (Fig. 1).
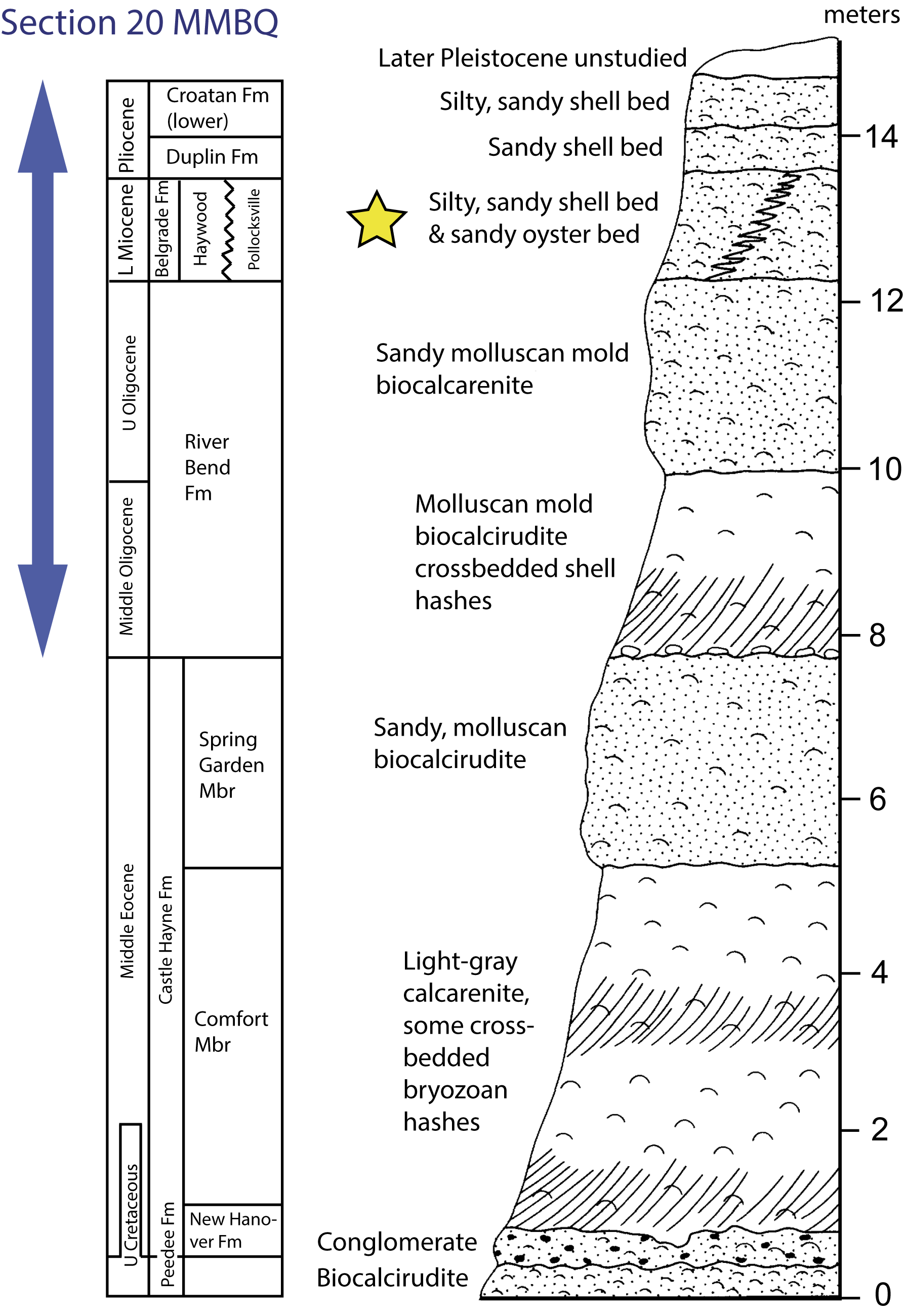
Figure 1. Composite stratigraphic section of Cenozoic formations from the Atlantic Coastal Plain, with the stratigraphic section from MMBQ (Locality 20), modified from Ward et al. (Reference Ward, Lawrence and Blackwelder1978). The in-situ location of the Maysville L.F. is indicated by the yellow star.
Within this local stratigraphic section, the fossil land mammals described here are presumed to come, or have been collected directly (i.e., during our field trips in 2018 and thereafter) from the Belgrade Formation. This 1.5-m-thick unconsolidated unit has two dominant arenaceous lithologies: (1) tan to orange somewhat leached oyster-bearing beds containing Striostrea gigantissima (Finch, Reference Finch1824) (sensu Bolton and Portell, Reference Bolton and Portell2013; although previously referred to the genus Crassostrea, see Lawrence, Reference Lawrence1975) of the Pollocksville Member; and (2) shell-rich phosphatic sands and clays of the Haywood Landing Member. Mostly because they are not collected in situ, it has been unclear which of the two recognized members yield the fossil land mammals. The stratigraphic and temporal interpretations and fossils contained in these two members of the Belgrade Formation have been debated, without consensus (e.g., Lawrence, Reference Lawrence1975; Baum et al., Reference Baum, Harris and Zullo1978; Ward et al., Reference Ward, Lawrence and Blackwelder1978).
In their description of the stratigraphic section at MMBQ, Ward et al. (Reference Ward, Lawrence and Blackwelder1978) recognized two Pliocene units (the Duplin and Croatan formations) overlying the Belgrade Formation. While fossil vertebrates are not formally described from either of these units at MMBQ (although see possible occurrences described in Ward et al., Reference Ward, Lawrence and Blackwelder1978, and Hipparion sp. described in Bruff et al., Reference Bruff, Bain, Chandler and Chandler2017), one horse tooth collected from this mine is morphologically too advanced to belong to the Maysville L.F., and too primitive and small to be a Pleistocene horse (i.e., Equus). This and some other land-mammal specimens that may come from post-Early Miocene sediments from MMBQ are also placed on record in this report (Appendix).
Previous paleontological discoveries at Martin Marietta Belgrade Quarry
The marine fossils and stratigraphic sequence exposed at MMBQ have figured prominently in understanding of the Cenozoic development of the Atlantic Coastal Plain of eastern North Carolina (Ward et al., Reference Ward, Lawrence and Blackwelder1978). Marine vertebrates include sharks and rays (Chondrichthyes), bony fish (Osteichthyes), whales and dolphins (Cetacea), dugongs (Sirenia), crocodiles (Crocodylia), and turtles (Testudines). Sharks and rays are by far the most abundant vertebrate fossils present. The problem with these taxa is that many of them are stratigraphically long-lived. Also, most have been recovered from spoil piles and therefore are of uncertain stratigraphic and temporal context.
Regarding fossil mammals, presumably from the Belgrade Formation, Boessenecker (Reference Boessenecker2022) described a diverse assemblage of whales and dolphins (cetaceans) and a fragmentary record of sirenians. These discoveries, primarily derived from surface prospecting of spoil piles, are attributed to the Belgrade Formation based on the stage of evolution of these taxa. Boessenecker (Reference Boessenecker2022) indicated that the cetaceans from the Belgrade Formation are transitional late Oligocene to Early Miocene in age (25.95–21.12 Myr). Over the past several decades, late Oligocene/Early Miocene land mammals have been recovered mostly from the mined spoil piles at MMBQ through occasional surface prospecting by professionals and citizen scientists (MacFadden et al., Reference MacFadden, Bohaska, McCall, Pirlo and Niederkorn2017; Perez et al., Reference Perez, Leder, Lundgren, Ellis, Dunckel and Crippen2020). MacFadden et al. (Reference MacFadden, Bohaska, McCall, Pirlo and Niederkorn2017) reported a preliminary list of land mammals from the Belgrade Formation including a rodent, entelodont (Daeodon), anthracothere, small selenodont artiodactyl (?protoceratid), peccary, horse (cf. Archaeohippus), and rhinoceros. This assemblage is assigned to the late Arikareean (Ar3/Ar4) to early Hemingfordian (He1) North American Land Mammal Ages (NALMA) and are described here as part of the Maysville L.F. Baskin et al. (Reference Baskin, Dickinson, DuBois, Galiano and Hartstone-Rose2020) described a left dentary with p4 from MMBQ as ?Amphictis, an ailurid carnivore. Given both the stratigraphic section in this mine (Fig. 1) and the stage of faunal evolution, at least 12 taxa of land mammals (Table 1) described below must come from the Belgrade Formation at MMBQ.
Table 1. Early Miocene land mammals and NALMA biochron from the Martin Marietta Belgrade Quarry, Jones County, North Carolina
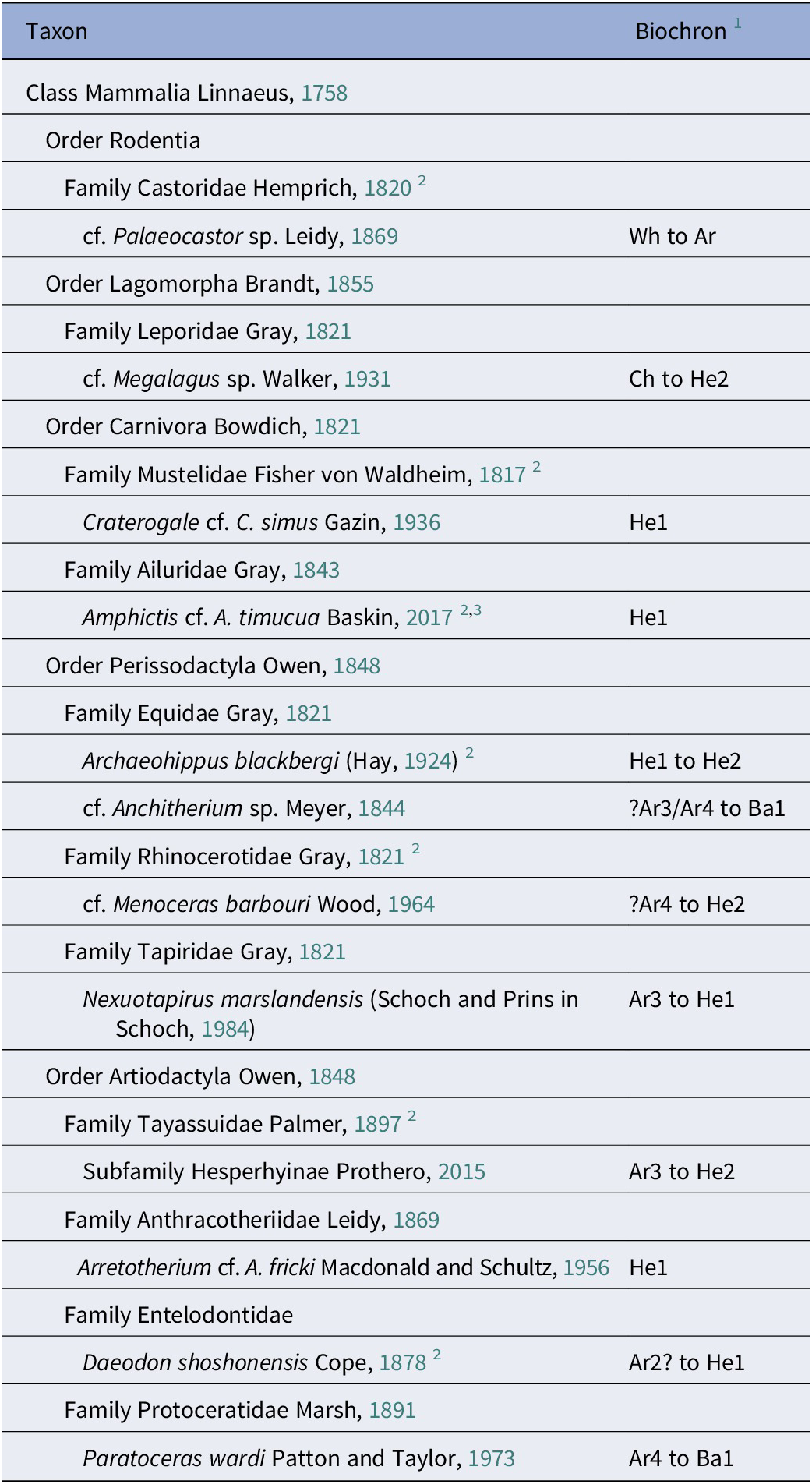
1 North American Land Mammal Age biochron, as taken from mid-latitude North America. See discussion, references, and rationale in text. Abbreviations (following Tedford et al., Reference Tedford, Albright, Barnosky, Ferrusquia-Villafranca, Hunt, Storer, Swisher, Voorhies, Webb, Whistler and Woodburne2004): Ar = Arikareean; Ba = Barstovian, Ch = Chadronian; He = Hemingfordian; Wh = Whitneyan. Some biochrons contain numerical subdivisions (e.g., Ar4, is the fourth and latest/youngest division of the Arikareean NALMA).
2 Also see Bruff et al. (Reference Bruff, Bain, Chandler and Chandler2017).
3 Also previously described by Baskin et al. (Reference Baskin, Dickinson, DuBois, Galiano and Hartstone-Rose2020).
Likewise, Bruff et al. (Reference Bruff, Bain, Chandler and Chandler2017), specifically mentioned fossils from MMBQ in their comprehensive monograph on fossil land mammals from North Carolina. These included specimens from the US National Museum of Natural History, North Carolina Museum of Natural Sciences, and private collections. Although lacking stratigraphic content like most of the Belgrade fossils, Bruff et al. (Reference Bruff, Bain, Chandler and Chandler2017) reported the following taxa from Belgrade (or Jones County, presumed here to be the same), including Camelidae, Rhinocerotidae, horses Archaeohippus and Hipparion, mustelid, peccary, Rodentia, “Euungulata,” Daeodon, and other unidentified small mammal teeth. Many of these are likely part of the Early Miocene Maysville L.F. described here, although some may come from the units overlying the Belgrade Formation at MMBQ (also see Appendix).
Strontium Isotope Stratigraphy (SIS), and Early Miocene marine–nonmarine correlations
Considering the abundance of fossil shark teeth at MMBQ, SIS using shark teeth was implemented to further resolve the ages for the Belgrade Formation, and thus the land mammals. This chronological tool can date specimens from marine environments and potentially clarify terrestrial mammal ages (NALMAs) for localities with marine and non-marine tie-ins (e.g., Tedford and Hunter, Reference Tedford and Hunter1984). This method in marine settings relies on globally uniform 87Sr/86Sr values throughout the oceans that change over geologic time due to different sediment fluxes and hydrothermal vent activity. 87Sr/86Sr ratios are correlated with the geologic timescale to produce the global strontium seawater curve (DePaolo and Ingram, Reference DePaolo and Ingram1985; Howarth and McArthur, Reference Howarth and McArthur1997; McArthur et al., Reference McArthur, Howarth, Shields, Zhou, Gradstein, Ogg, Schmitz and Ogg2020). Marine organisms readily incorporate strontium into their skeletons from the oceans while living, thereby producing a timestamp when correlated to the global seawater curve. While carbonate invertebrate fossils are the traditional source material for SIS (DePaolo and Ingram, Reference DePaolo and Ingram1985), recent studies have demonstrated that the outer durable layer of enameloid from shark teeth provides accurate age correlations as well (Becker et al., Reference Becker, Seidemann, Chamberlain, Buhl and Slattery2008; Bosio et al., Reference Bosio, Bianucci, Collareta, Landini, Urbina and Di Celma2022; Killingsworth, Reference Killingsworth2023).
The Early Miocene exemplifies a time of global changes in marine Sr ratios, and consequently, displays a steeper slope on the global seawater curve, making it an ideal point in time to utilize SIS (Hodell et al., Reference Hodell, Mueller and Garrido1991; Hodell and Woodruff, Reference Hodell and Woodruff1994). Sugarman et al. (Reference Sugarman, Miller, Owens and Feigenson1993) used SIS to confirm the age of Early Miocene deposits from the Kirkwood Formation of the New Jersey Coastal Plain. Those results aligned with the geological time scale as well as the diatom-based biostratigraphy for the area. The integration of SIS thus provided additional chronological evidence and comparisons with eustatic sea level changes and tectonics for the New Jersey Coastal Plain. Similarly, Bosio et al. (Reference Bosio, Bianucci, Collareta, Landini, Urbina and Di Celma2022) successfully used SIS of shark teeth from the Miocene Chilcatay Formation in the Pisco Basin of southwestern Peru to demonstrate their accuracy for providing age calibrations. The shark tooth SIS results were consistent with a Middle to Late Miocene age (14.8–6.7 Myr) for their study area, which is also based on biostratigraphy and tephrochronology. In the case of the Oligo-Miocene faunas of MMBQ, our new SIS results inform longstanding questions about the paleogeography and eustatic changes, as well as clarify the relative ages of the members of the Belgrade Formation at this locality.
Materials and methods
Field work
Fossil specimens described here have been collected mostly by surface prospecting of spoil piles over the past few decades, both by paleontologists from several institutions and dedicated citizen scientists (Perez et al., Reference Perez, Leder, Lundgren, Ellis, Dunckel and Crippen2020). Prior to 2018, all specimens were collected from spoil piles and thus lack demonstrable provenience. Based on the stratigraphic section exposed at MMBQ, these fossil vertebrates could be mixed from any of the units, including the River Bend, Belgrade, Duplin, and Croatan formations, as well as unstudied Pleistocene cover (Fig. 1).
In 2018, the miners sequestered part of the in-situ Belgrade Formation into piles for fossil collecting, primarily in support of this research project. These piles were then surface prospected by groups of collectors on day-long trips to MMBQ over the next several years until storms completely flooded and washed away large areas of the mine, including the in-situ fossil piles. Prior to that time, approximately 500 kg of the in-situ Belgrade sediments were screenwashed for microfauna. While these combined efforts have resulted in thousands of fossils, they are almost exclusively marine (chondrichthyans and osteichthyans). Specimens of invertebrates, including Striostrea and Chione, were collected for geochemical analyses from a separate set of sequestered piles of the Belgrade Formation on May 19, 2023.
Systematic paleontology
The classification sequence of higher taxonomic categories (Order and above) follows O’Leary et al. (Reference O’Leary, Bloch, Flynn, Gaudin and Giallombardo2013). For Family within Order, we follow McKenna and Bell (Reference McKenna and Bell1997). All specimens described below (except as noted) come from MMBQ, Jones County, North Carolina. These were collected from float (mined spoil piles), presumed Belgrade Formation, herein designated as the Maysville L.F., Early Miocene, and likely span the late Arikareean–early Hemingfordian NALMAs. We also report on land mammals from MMBQ that are too advanced in their evolution to be from the Maysville L.F. (see Appendix).
Sr-isotope stratigraphy
Sampling
For the vertebrates, seven well-preserved specimens of shark tooth enameloid (genera Hemipristis and Carcharias) were each sampled three times from different areas on the tooth crown. For the invertebrates, four clam specimens (genus Chione) were each sampled three times from different areas along the dorsal, anterior, and ventral margins. Likewise, four oyster specimens (genus Striostrea) were each sampled three times along the hinge.
Preparation
A rotary drill with a dental-grade carbide tip was used to collect powdered samples ranging from 11.0–19.2 mg, depending on tooth size. Samples were prepared and processed in a clean lab following the UF Department of Geological Sciences protocol as follows: Samples (100 μl) were loaded in cation-exchange columns packed with strontium-specific resin (El Chrom Part #SR-B100-S) and rinsed four times with 100 μl of 3.5 HNO3, followed by a final 1 ml 3.5 HNO3 wash. Strontium was collected in refluxed Teflon vials with 1.5 ml of 4XH2O. The samples were analyzed on a Nu Plasma multiple collector inductively coupled mass spectrometer in the Department of Geological Sciences at the University of Florida, comparing to the NBS987 control standard run every third or fourth pass. The raw data were corrected for mass bias, Rb presence, and any gas interference and converted to age using the Version 4 Table (Howarth and McArthur, Reference Howarth and McArthur1997; McArthur et al., Reference McArthur, Howarth, Shields, Zhou, Gradstein, Ogg, Schmitz and Ogg2020).
Analyses
Statistical analyses were done in R (R Studio Team, 2020; R Core Team, 2022). Sr-corrected values for each sample were calibrated to the GTS2020 timescale using the LOWESS Version 4 Look-Up Table to determine their preferred ages (Howarth and McArthur, Reference Howarth and McArthur1997; McArthur et al., Reference McArthur, Howarth, Shields, Zhou, Gradstein, Ogg, Schmitz and Ogg2020). Cumulative means from the preferred ages for the three samples per specimen were then calculated and a final overall mean taken.
Repositories, databases, and institutional abbreviations
Specimens described here are primarily housed at the National Museum of Natural History, Washington, D.C. (USNM acronym used for US National Museum of Natural History, Department of Paleobiology collections). Additional specimens are housed in the Carnegie Museum of Natural History (CM), Florida Museum of Natural History (University of Florida, acronym UF for Florida Museum Vertebrate Paleontology Collection), Louisiana State University Museum of Geology (LSUMG), North Carolina Museum of Natural Sciences (NCSM), and Princeton University (PU; now part of Yale Peabody Museum). Two specimens described in this paper (USNM 639766, 639777) are represented by plaster casts, and one (UF 552612) by a high-resolution 3D printed replica. These vouchered replicas were studied because the original specimens are in private collections and currently not available for study. Five catalogued specimens (USNM 629773, 639762–639764, 639768) from MMBQ in the USNM collections were not studied here because they mostly represent fragmentary or otherwise unidentifiable skeletal elements. Digital data for some specimens described here are retrievable from the Integrated Digitized Biocollections (https://www.idigbio.org) and the Global Biodiversity Information Facility (https://www.gbif.org). The biochronological ranges of these taxa were retrieved from original literature, and from the Paleobiology Database (https://paleobiodb.org).
Systematic paleontology
Class Mammalia Linnaeus, Reference Linnaeus1758
Order Rodentia Bowdich, Reference Bowdich1821
Family Castoridae Hemprich, Reference Hemprich and Hemprich1820
Genus Palaeocastor Leidy, Reference Leidy1869
Type species
Palaeocastor nebrascensis (Leidy, Reference Leidy1856).
cf. Palaeocastor sp.
Figure 2.1, 2.2, Tables 1, 2
Table 2. Measurements (mm) of teeth and postcranial elements for the land mammals of the Maysville L.F. from MMBQ.
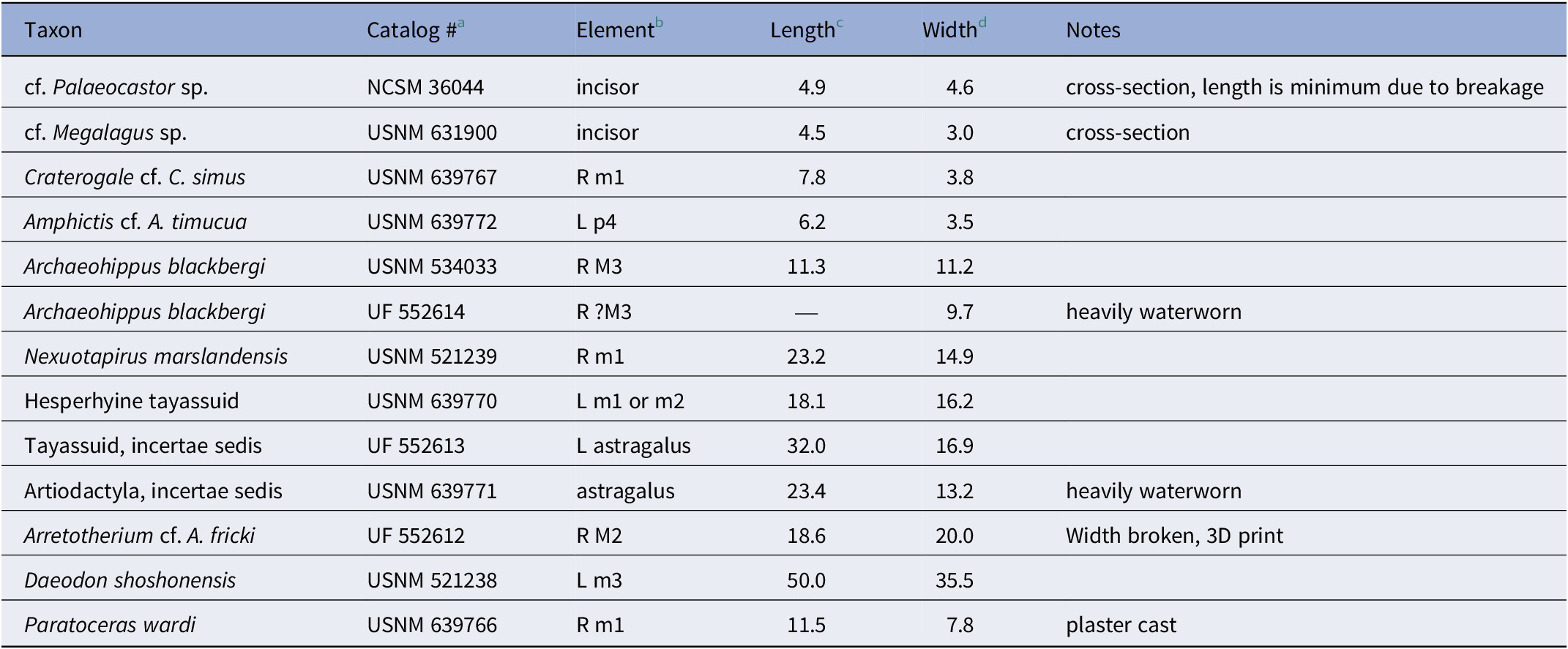
a See ‘repositories, databases, and institutional abbreviations’ section in text.
b see tooth position abbreviations in text.
c for teeth this represents the greatest anteroposterior length (L) except for cf. Palaeocastor sp. and cf. Megalagus sp., in which this measurement represents cross-sectional anteroposterior length; for postcranial elements it represents the greatest proximal–distal length.
d for teeth this represents the greatest (transverse) width (W) except for Castoridae and cf. Megalagus sp., which represent cross-sectional transverse/mesiodistal width; for postcranial elements it represents the greatest medial–lateral (transverse) width.
Occurrence
The genus Palaeocastor ranges from the Whitneyan (Oligocene) through the Arikareean (Early Miocene) in North America (Korth, Reference Korth1994; Flynn and Jacobs, Reference Flynn, Jacobs, Janis, Gunnell and Uhen2008; https://paleobiodb.org).
Materials
NCSM 36044, left lower incisor (di2), collected by Terry Denny; Garner, North Carolina (abbreviation NC; also, NC Fossil Club).
Remarks
The incisor preserves the apical portion of the tooth with damage to the dentine of the lingual surface along much of its length (Fig. 2). The enamel band barely extends onto the mesial surface of the tooth, although grades, almost indistinguishably, into the dentine on the distal surface. Along the enameled buccal aspect of the tooth are several faint, but recognizable, longitudinal ridges. This differs from faint latitudinal texturing in lagomorph teeth, supporting referral to Rodentia. Given the damage to the lingual surface of the incisor, it is difficult to interpret the cross-sectional morphology. However, where preserved, the mesial, buccal, and distal faces intersect at ~90° angles resulting in a nearly square cross-section at its base (i.e., a flat anterior surface sensu Rybczynski, Reference Rybczynski2007). The curvature radius (Skinner and Taylor, Reference Skinner and Taylor1967) of the tooth is ~21 mm, supporting the identification of a left lower incisor. The measurements presented here (Table 2) fall within those reported by Korth and Samuels (Reference Korth and Samuels2015) for species of Palaeocastor. Given the morphology of the incisor, particularly with its relatively flat anterior surface (Korth, Reference Korth1994) and relative size, this tooth most likely represents a castorid that we refer to here as cf. Palaeocastor sp.
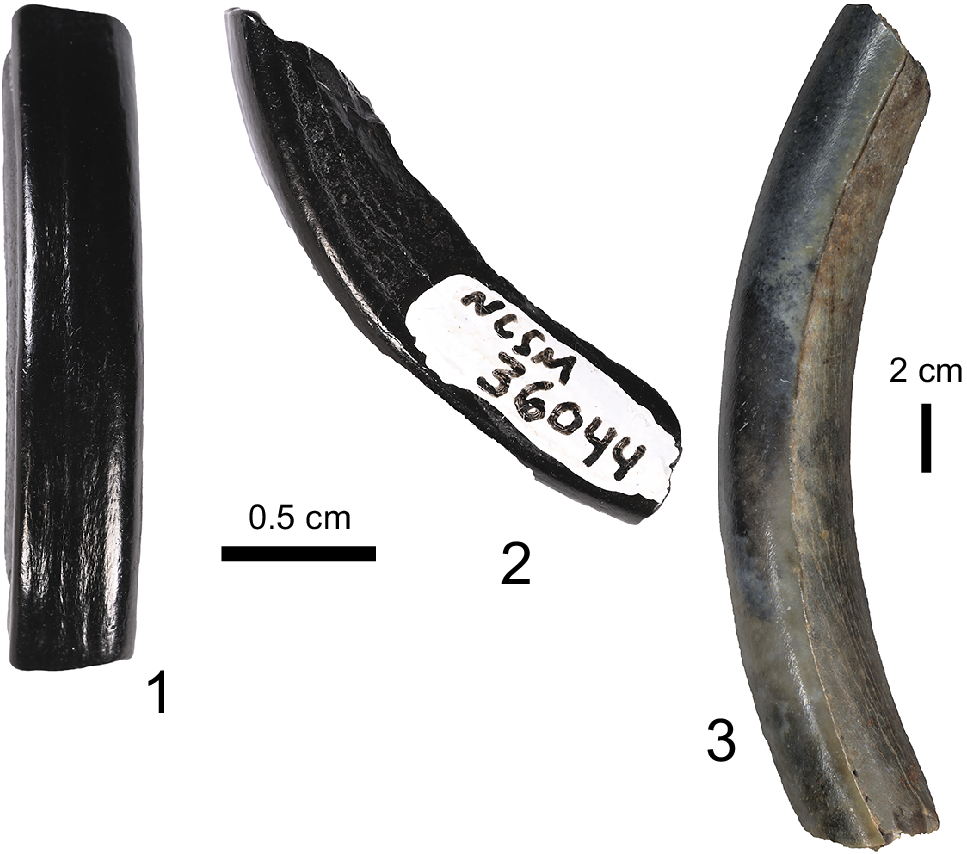
Figure 2. Glires incisors from the Maysville L.F. NCSM 36044, cf. Palaeocastor sp.: (1) buccal; (2) distal. (3) USNM 631900, stem Lagomorpha, cf. Megalagus sp. lateral.
Order Lagomorpha Gidley, Reference Gidley1912
Genus Megalagus Walker, Reference Walker1931
Occurrence
The genus Megalagus has a long biochronological range from the late Eocene (Chadronian) to the Middle Miocene (He2) in North America (Dawson, Reference Dawson, Janis, Gunnell and Uhen2008). After the late Oligocene, however, the distribution of Megalagus may be restricted primarily to relictual occurrences in the Gulf Coastal Region (Janis et al., Reference Janis, Dawson, Flynn, Janis, Gunnell and Uhen2008).
Materials
USNM 631900, partial lower ?R incisor (di2), collected by George Fonger.
Previous reference
Rodent, MacFadden et al. (Reference MacFadden, Bohaska, McCall, Pirlo and Niederkorn2017).
Remarks
Isolated incisors of small mammals are oftentimes very difficult to identify taxonomically, and therefore, they are not typically discussed much in the literature, even in classic monographs (e.g., Wood, Reference Wood1940; Dawson, Reference Dawson1958). Nevertheless, this tooth (Fig. 2.3) has characteristic smooth enamel on the external surface, and a curvature radius (Skinner and Taylor, Reference Skinner and Taylor1967) of 50 mm. The enamel covers the entire buccal surface and extends along part of the mesial and distal surfaces of the tooth. The specimen is broken so that the sharp edge (apex), which is characteristic of ever-growing incisors of Glires, is not preserved. Likewise, the posterior part of the tooth is not preserved, but the cross-section (Table 2) is hollow, likely where the pulp would have been during life, and the open root is characteristic of hypselodonty. USNM 631900 is considerably larger than Palaeolagus but compares favorably in size and smooth enamel structure with Megalagus (UF 505912), as both of these genera are represented by UF collections from the Brule Formation (Orellan) of the Nebraska badlands. Traditionally, Megalagus has been included as a primitive genus within the Family Leporidae (e.g., Dawson, Reference Dawson1958). More recently, however, López-Torres (Reference López-Torres, Bertrand, Lang, Silcox and Fostowicz-Frelik2020) reinterpreted Megalagus as a stem lagomorph, lying outside of the Family Leporidae.
Order Carnivora, Bowdich, Reference Bowdich1821
Family Mustelidae Fischer von Waldheim, Reference Fischer von Waldheim1817
Genus Craterogale Gazin, Reference Gazin1936
Type species
Craterogale simus Gazin, Reference Gazin1936.
Craterogale cf. C. simus Gazin, Reference Gazin1936
Figure 3.1, 3.2, Tables 1, 2
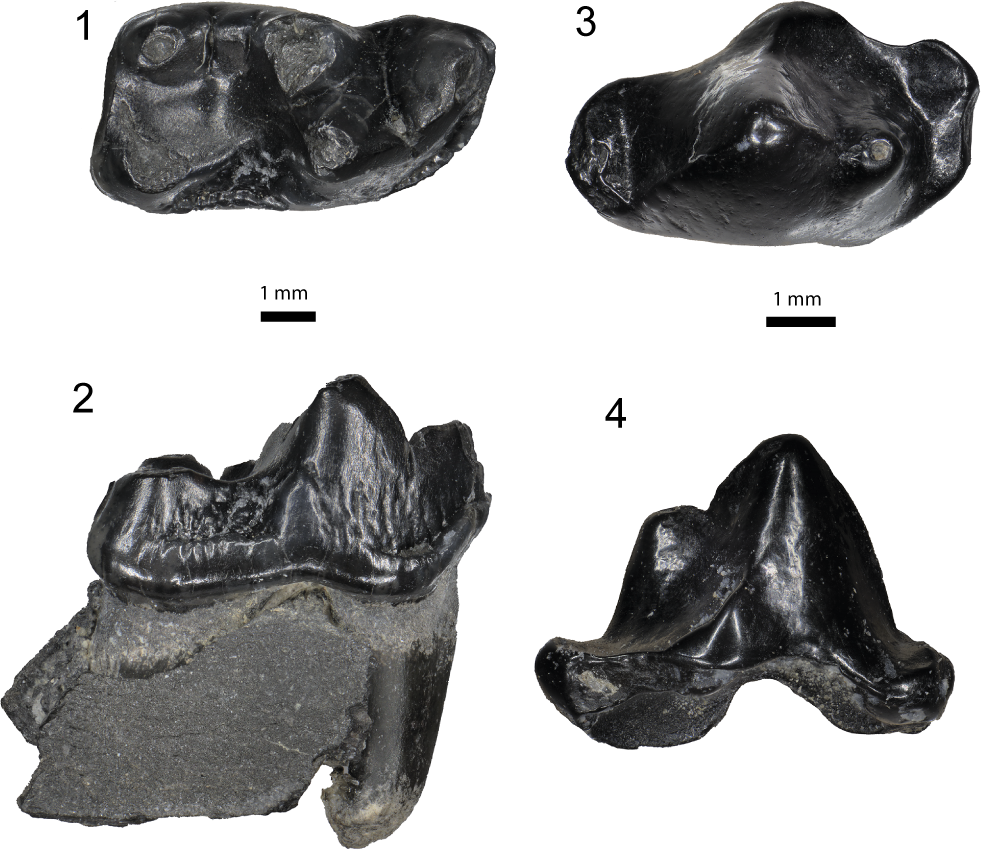
Figure 3. Carnivora from the Maysville L.F. (1, 2) Craterogale cf. C. simus (Mustelidae), USNM 639767, right dentary fragment with m1: (1) occlusal; (2) buccal. (3, 4) Amphictis cf. A. timucua (Ailuridae), USNM 639772, left p4: (3) occlusal, anterior to left; (4) buccal.
Holotype
Nearly complete skull and posterior upper cheek tooth dentition (USNM 13801), from Antelope Valley, Dawes County, Nebraska, Miocene (Gazin, Reference Gazin1936).
Diagnosis
See Gazin (Reference Gazin1936) for the holotype, as emended by Baskin (Reference Baskin2017) for the lower dentition.
Occurrence
Runningwater Formation (type locality), Nebraska, and UF Miller site (DI002), Florida, both He1.
Description
The m1 is well preserved. The protoconid is the highest cusp in the tooth. The paraconid is situated just lingual to the midline. A narrow slit on the buccal margin prevents closure of the valley between the protoconid and paraconid. The metaconid is well developed lingually, although its tip is worn. A deep talonid basin is formed posteriorly with a cristid that runs between the metaconid and entoconid, and a well-developed (although worn or broken) postcristid connects the entoconid and hypoconid. The posterior hypoconulid is faint. A cingulid is well developed buccally and the enamel of the buccal side of the tooth is distinctly crenulated. No cingulid is developed lingually.
USNM 639767 from MMBQ is a small carnivoran, with m1 L of 7.8 mm and W of 3.8 mm. It is most similar to, although slightly smaller than (mean m1 L = 8.9 mm, range = 8.3– 9.5 mm; mean m1 W = 4.4 mm, range = 4.2–4.9 mm) the four comparable specimens referred to Craterogale cf. C. simus from Nebraska and Florida (Baskin, Reference Baskin2017, table 6).
Materials
USNM 639767, right dentary fragment with m1 (Fig. 3.1, 3.2), collected by Alex Bagster-Collins.
Previous reference
Tooth in jaw fragment, Bruff et al. (Reference Bruff, Bain, Chandler and Chandler2017, p. 206).
Remarks
Gazin (Reference Gazin1936) described the genus and species Craterogale simus Gazin, Reference Gazin1936, a leptarctine mustelid, based on a nearly complete skull with upper dentition, lacking only the rostral portion and anterior dentition (incisors, canine, and anterior premolars) from the Hemingfordian of Dawes County, Nebraska. In describing the carnivorans from the Miller Site in Florida, Baskin (Reference Baskin2017) referred several specimens to Craterogale cf. C. simus, including two separate dentaries with the m1. USNM 639767 from the Maysville L.F. is of similar size and dental morphology to those specimens from the Miller Site. Baskin (Reference Baskin2017) indicated a Hemingfordian age for the Miller Site, similar to the biochron of the Runningwater Formation from the type locality in Nebraska. Bruff et al. (Reference Bruff, Bain, Chandler and Chandler2017) previously illustrated USNM 639767, but with no accompanying description.
Family Ailuridae Gray, Reference Gray1843
Genus Amphictis Pomel, Reference Pomel1853
Type species
Amphictis antiqua (de Blainville, Reference de Blainville1842).
Amphictis cf. A. timucua Baskin, Reference Baskin2017
Figure 3.3, 3.4, Tables 1, 2
Holotype
Left dentary with p3–m1 (UF 246332), Miller Site, northern Florida, Early Miocene (Baskin, Reference Baskin2017).
Diagnosis
Of the diagnostic characters within the genus Amphictis, size is the most relevant here, with A. timucua being the smallest species (Baskin, Reference Baskin2017).
Occurrence
According to Tedford et al. (Reference Tedford, Albright, Barnosky, Ferrusquia-Villafranca, Hunt, Storer, Swisher, Voorhies, Webb, Whistler and Woodburne2004), the first occurrence of Amphictis is one of the defining taxa at the base of the Hemingfordian NALMA, and occurs throughout He1 (Baskin, Reference Baskin2017). Amphictis cf. A. timucua is the only known species of this genus from North America of relevance here. It has previously been described from Florida (https://paleobiodb.org) and MMBQ (Baskin et al., Reference Baskin, Dickinson, DuBois, Galiano and Hartstone-Rose2020).
Description
USNM 639772 represents a well-preserved crown of a small carnivoran with smooth enamel (Fig. 3.3, 3.4; Table 1, 2). The anteroposterior dimension of the tooth is almost twice as long as the transverse (buccolingual) width. The perimeter is characterized by anterior and posterior cingulids. An anterior cuspid is large, transversely situated in the middle of the tooth, and forms the highest part in buccal view. A smaller cuspid is posterobuccal to the anterior cuspid and merging into the former via a weakly developed lophid. No other cuspids or stylids are developed.
Materials
USNM 639772, left p4 crown, lacking roots (Fig. 3.3, 3.4), collected by Julie Niederkorn; Cary, NC (NC Fossil Club).
Previous reference
Tooth, Bruff et al. (Reference Bruff, Bain, Chandler and Chandler2017, p. 205); ?Amphictis Baskin et al., Reference Baskin, Dickinson, DuBois, Galiano and Hartstone-Rose2020.
Remarks
In his description of A. timucua, Baskin (Reference Baskin2017) reviewed the eight previously named valid species assigned to Amphictis, seven of which are from Eurasia. He named A. timucua from the early Hemingfordian (He1) of Florida. Along with the holotype (UF 246332), the hypodigm also includes another well-preserved dentary with p3 and p4 (UF 246350). Baskin et al. (Reference Baskin, Dickinson, DuBois, Galiano and Hartstone-Rose2020) also previously described a dentary referred to ?Amphictis (NCSM 33670) from MMBQ, presumably from the Belgrade Formation. All three of these prior specimens preserve the p4, which allows comparisons with the Belgrade tooth described here, corroborating both the general dental morphology and similar size of these specimens. In A. timucua from Florida and the previously described ?Amphictis from MMBQ, the lingual margin of these teeth forms a relatively smooth continuous curve. However, in USNM 639772 the middle of the tooth projects lingually, resulting in anterior and posterior (concave) reentrants in the position of, respectively, the trigonid and talonid regions. This morphological difference is not considered to be taxonomically significant given the currently available small population with which to assess the variation of this character.
In a prior assessment of the biochronology of ?Amphictis from Belgrade, Baskin et al. (Reference Baskin, Dickinson, DuBois, Galiano and Hartstone-Rose2020) considered the presence of this taxon to indicate an earlier range extension (i.e., prior to the earliest Hemingfordian). This extension followed the preliminary biochronological assessment of MacFadden et al. (Reference MacFadden, Bohaska, McCall, Pirlo and Niederkorn2017). Nevertheless, based on the other biochronological evidence presented here for the age of the Maysville L.F., the presence of Amphictis, and more specifically A. cf. A. timucua, may not need to be explained as earlier range extension. Thus, the first-occurrence dispersal of Amphictis into North America posited by Tedford et al. (Reference Tedford, Albright, Barnosky, Ferrusquia-Villafranca, Hunt, Storer, Swisher, Voorhies, Webb, Whistler and Woodburne2004) may still be considered an index taxon for early Hemingfordian time based on its presence in the Maysville L.F. Amphictis is thus important biochronologically as an Eurasian immigrant during the Runningwater Chronofauna.
Order Perissodactyla, Owen, Reference Owen1848
Family Equidae Gray, Reference Gray1821
Genus Archaeohippus Gidley, Reference Gidley1906
Type species
Archaeohippus ultimus Gidley, Reference Gidley1906.
Archaeohippus blackbergi (Hay, Reference Hay1924)
Figure 4.1, 4.2; Tables 1, 2
Holotype
Left M3, (Texas Memorial Museum) TMM 31084-2376, formerly (Texas A&M College Museum) TAM 2376, from Garvin Gully, Texas, Miocene (Hay, Reference Hay1924).
Occurrence
Hemingfordian (both He1 and He2) of Texas, South Dakota, Florida, and likely Delaware (Hulbert, Reference Hulbert2015; https://paleobiodb.org).
Description
USNM 534033 is a well-preserved tooth in early wear stage (Fig. 4.1); UF 552614 preserves the buccal half of the molar (Fig. 4.2), which is of uncertain position in the tooth row. These two specimens demonstrate characters of Archaeohippus blackbergi (Stirton, Reference Stirton1941). These include relatively small size (Table 2), brachydont cheek teeth, metaloph connected to the ectoloph intersecting the mesostyle, ribs and styles well developed on the ectoloph, and lacking a crochet. The ectolophs contain distinct parastyles, metastyles, and a relatively smaller mesostyle. Four principal cones are distinct from the lophs (i.e., the paraconule and protocone on the protoloph and the metacone and hypocone on the metaloph). The lophs form distinct transverse crests. The hypostyle is developed posterior to the metaloph. USNM 534033 lacks cingula on the anterior, posterior, or buccal sides of the tooth, except lingually where a cingulum is faint. Regarding the size of USNM 534033, the transverse width of 11.2 mm (Table 2) falls within the observed range of 9.0–11.8 mm (mean width [W] 10.99 ± 0.21 mm) reported for a population of 13 M3s of A. blackbergi from Thomas Farm (Bader, Reference Bader1956; Table 2).
Materials
USNM 534033, RM3, collected by Frederick Grady (USNM); UF 552614, R ?M3, collected by Eric Sadorf; Cary, NC (NC Fossil Club).
Previous reference
Archaeohippus sp. Bruff et al. (Reference Bruff, Bain, Chandler and Chandler2017); cf. Archaeohippus, MacFadden et al. (Reference MacFadden, Bohaska, McCall, Pirlo and Niederkorn2017).
Remarks
These two teeth compare favorably with large samples of A. blackbergi in the UF collections from Thomas Farm (e.g., White, Reference White1942; Bader, Reference Bader1956), as well as smaller, but also representative, samples of A. cf. A. blackbergi from the Pollack Farm L.F. of Delaware (Emry and Eshelman, Reference Emry, Eshelman and Benson1998). Isolated teeth of extinct equid species are typically difficult to identify. Nevertheless, using the better-preserved specimen from MMBQ (USNM 534033), at first glance it would seem to pertain to a species of Archaeohippus smaller than A. blackbergi (e.g., A. mannulus, O’Sullivan, Reference O’Sullivan2003). However, once the tooth position is definitively identified as M3 for USNM 534033, then an unambiguous identification of A. blackbergi can be made, in which this tooth is distinctly smaller than the other upper cheek teeth of this species, as both White (Reference White1942) and Hulbert (Reference Hulbert2015) also noted. This assignment is also confirmed by comparisons with more complete materials (e.g., the complete cheek tooth dentition of A. blackbergi, as exemplified by UF 156481, from Thomas Farm, Florida).
The sample of Archaeohippus documented above consists of two isolated teeth, one complete (USNM 534033) and one fragmentary (UF 552614). In addition, Bruff et al. (Reference Bruff, Bain, Chandler and Chandler2017, p. 189) illustrated a partial maxilla with two cheek teeth referred to Archaeohippus sp. from Jones County. The MMBQ is in Jones County and although the exact location is not indicated, it is likely that this associated dentition came from the Belgrade Formation and represents A. blackbergi. The disposition of this specimen is uncertain and therefore not available for examination during this study. The definitive presence of A. blackbergi would indicate a Hemingfordian (Tedford et al., Reference Tedford, Albright, Barnosky, Ferrusquia-Villafranca, Hunt, Storer, Swisher, Voorhies, Webb, Whistler and Woodburne2004; Hulbert, Reference Hulbert2015) age for the Maysville L.F.
Genus cf. Anchitherium Meyer, Reference Meyer1844
cf. Anchitherium sp.
Type species
Anchitherium ezquerre (Meyer, Reference Meyer1844).
Occurrence
In North America, the genus Anchitherium occurs during the Early and Middle Miocene from the late Arikareean (?Ar3/Ar4) to the early Barstovian (Ba1; MacFadden, Reference MacFadden, Janis, Scott and Jacobs1998; https://paleobiodb.org).
Materials
USNM 639765, fragmentary premolar, collected by Peter Harmatuk, NC Fossil Club.
Remarks
This tooth fragment is so heavily worn and fragmentary (Fig. 4.3, 4.4) that a definitive taxonomic attribution is a challenge, as is the tooth position. The fragment has two lophs on the side of the tooth with a persistent and well-developed cingulum, which narrows down suitable candidates and cheek tooth position. In these characters USNM 639765 compares favorably with premolars of Anchitherium clarencei Simpson, Reference Simpson1932, from Thomas Farm, Florida.
USNM 639765 is so fragmentary and in such advanced wear that it cannot confidently be identified as an upper or lower premolar. Furthering uncertainty, the color of the preserved fossil, with the light brown enamel, and brown dentine and roots, is different from most of the other teeth from the Belgrade Formation, the latter of which are typically dark gray or brown. While the significance of this color difference is noteworthy, without further information it does not alter either the taxonomic or biochronological assignments of USNM 639765.
Family Rhinocerotidae, Gray, Reference Gray1821
Genus Menoceras Troxel, Reference Troxell1921
Type species
Menoceras arikarense Troxell, Reference Troxell1921.
cf. Menoceras barbouri Wood, Reference Wood1964
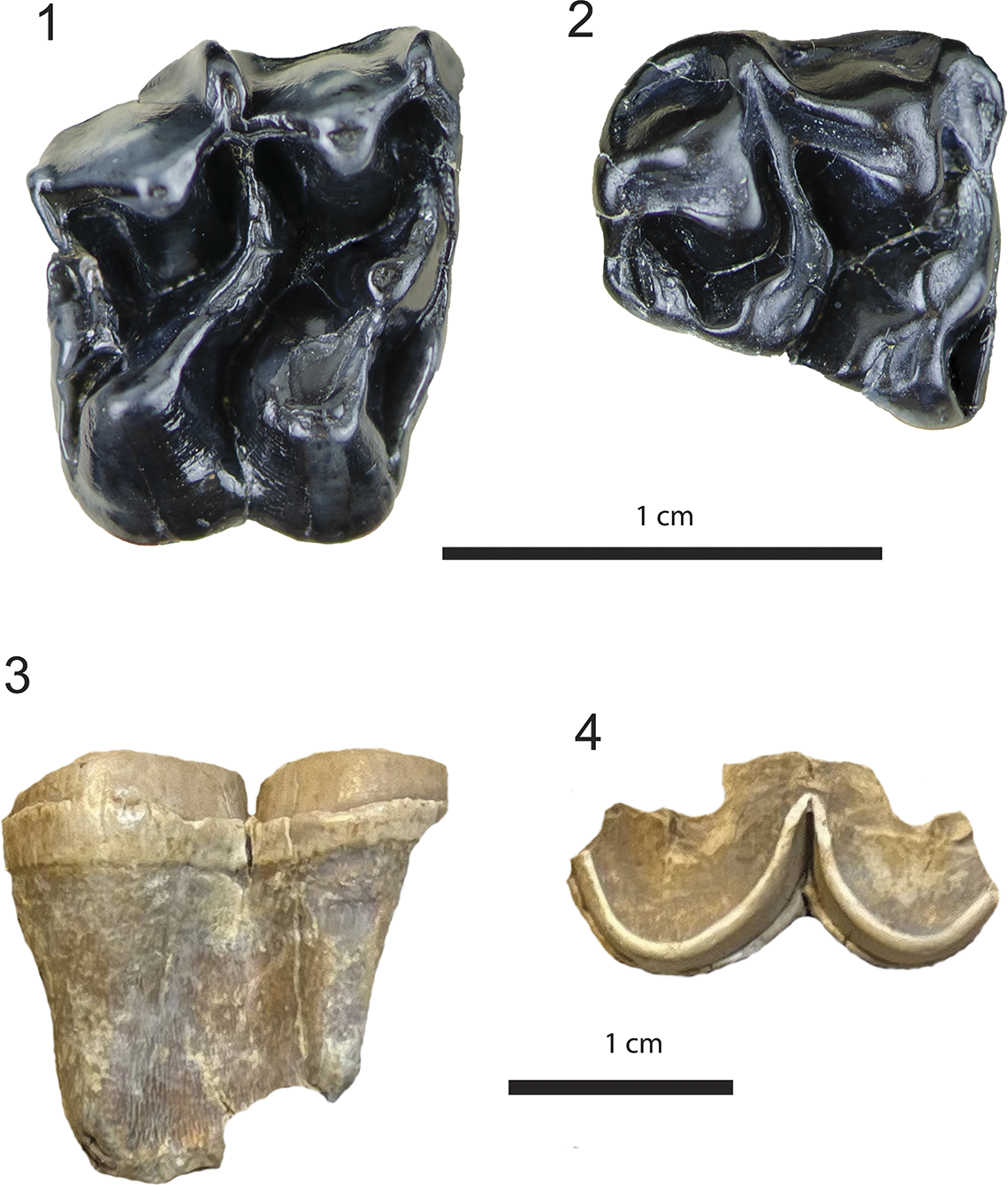
Figure 4. Equidae from the Maysville L.F. Archaeohippus blackbergi: (1) USNM 534033, right M3 (left), occlusal; (2) UF 552614, partial right ?M3, occlusal. cf. Anchitherium sp., USNM 639765, premolar fragment (3) buccal, (4) occlusal.

Figure 5. cf. Menoceras barbouri (Rhinocerotidae), USNM 639774, unciform, from the Maysville L.F.: (1) anterior (external) view with part of the articular surface for the cuneiform; (2) posterior view showing the diagnostic posteriorly directed unciform process; (3) proximal view with the cuneiform and lunar articular surfaces, with the position of the magnum articular surface (not shown); (4) distal view showing the articular surfaces for the MC III and MC IV. Abbreviations: cu = cuneiform; lu = lunar; mg = magnum; mc = metacarpal; up = unciform process.
Holotype
Menoceras barbouri, palate, basicranium, and occiput, MCZ 4452 (Museum of Comparative Zoology), from the Early Miocene Thomas Farm, Florida (Wood, Reference Wood1964).
Occurrence
Menoceras barbouri is widespread in North America (including Panama) and spans the late Arikareean (Ar4) to late Hemingfordian (He2) NALMAs (Janis et al., Reference Janis, Colbert, Coombs, Lambert, MacFadden, Mader, Prothero, Schoch, Shoshani, Wall, Janis, Scott and Jacobs1998; Prothero, Reference Prothero, Janis, Scott and Jacobs1998; https://paleobiodb.org, 2023).
Description
This specimen has the diagnostic morphology of carpal elements seen in fossil rhinos in the UF collection and described in the literature (e.g., Prothero and Manning, Reference Prothero and Manning1987; also see Short et al., Reference Short, Wallace and Emmert2019, for Teleoceras). Anteriorly, USNM 639774 (Fig. 5.1) shows a rugose bony surface forming part of the external carpus, the unciform process, and the side of the cuneiform facet. Posteriorly (Fig. 5.2), a well-developed rugose unciform process extends posteriorly, and articular processes for the fourth metacarpal. In the proximal aspect (Fig. 5.3), on the anterior side there are articular facets for the cuneiform and lunar. In distal aspect (Fig. 5.4) articular surfaces for the third and fourth metacarpals are developed.
Materials
USNM 639774, right carpal element (unciform), collected by Julie Niederkorn; Cary, NC (NC Fossil Club).
Previous reference
Rhinocerotidae sp., Bruff et al., Reference Bruff, Bain, Chandler and Chandler2017; rhino, MacFadden et al., Reference MacFadden, Bohaska, McCall, Pirlo and Niederkorn2017.
Remarks
Although morphologically similar, USNM 639774 is larger than unciforms of Subhyracodon occidentalis (Leidy, Reference Leidy1850) (UF 547406) from the lower Brule Formation (Orellan), Nebraska, and smaller than Floridaceras whitei Wood, Reference Wood1964 (UF 11239, UF 19856) from the Hemingfordian Thomas Farm site in Florida (Wood, Reference Wood1964). Unfortunately, no unciforms exist for the other rhino from Thomas Farm, Menoceras barbouri, for which this is the type locality (Wood, Reference Wood1964). In relative size, USNM 639774, although slightly smaller, compares most favorably with specimens in the UF collections assigned to Menoceras barbouri, including UF 223580 from the early Hemingfordian (He1) Cucaracha Formation of Panama.
Bruff et al. (Reference Bruff, Bain, Chandler and Chandler2017) also illustrated a carpal bone attributed to Rhinocerotidae sp. from MMBQ. Their photo (Bruff et al., Reference Bruff, Bain, Chandler and Chandler2017, p. 183) is likely USNM 639774.
Family Tapiridae Gray, Reference Gray1821
Genus Nexuotapirus Albright, Reference Albright1998
Type species
Nexuotapirus marslandensis (Schoch and Prins in Schoch, Reference Schoch1984).
Nexuotapirus marslandensis (Schoch and Prins in Schoch, Reference Schoch1984)

Figure 6. Nexuotapirus marslandensis (Tapiridae) from the Maysville L.F., USNM 521239, right m1 (1) occlusal (anterior to right), (2) buccal.
Holotype
Left partial dentary with M1 and M2 (AMNH 82804), from 29 km east of Agate, Sioux County, Nebraska. Exact locality unknown, although probably from the Runningwater Formation near Marsland (Albright, Reference Albright1998).
Diagnosis
Of relevance to the specimen from MMBQ, lower molars of Nexuotapirus marslandensis are characterized by a relatively complex dental pattern with a cuspid extending posteriorly from the protolophid (Albright, Reference Albright1998).
Occurrence
Early late Arikareean (presumably Ar3) to early Hemingfordian (He1) in Texas, Colorado, South Dakota, and Oregon (Albright, Reference Albright1998).
Description
The tooth from Belgrade is characteristically tapiroid, with well-developed crests and lophids, including in the trigonid, the paralophid, and the prominent transverse protolophid, which connects the protoconid and metaconid (Fig. 6.1). In the talonid, there is a well-developed metaloph and transverse hypolophid, the latter of which connects the hypoconid and entoconid. The diagnostic cuspid mentioned above is relatively well developed. Anteriorly the tooth has a well-developed cingulid; posteriorly there is a prominent hypoconulid.
Materials
USNM 521239, right m1, collected by Jodie McDaniel.
Previous reference
Possibly Tapiravus sp., tooth, Onslow County (Bruff et al., Reference Bruff, Bain, Chandler and Chandler2017, p. 181).
Remarks
Although USNM 521239 from Belgrade has a less-worn occlusal surface, it compares favorably in size (Table 2) and dental pattern to the more heavily worn specimen, LSUMG V-2263 (Albright, Reference Albright1998, fig. 9C), the latter of which consists of a partial dentary with left m1–m2, and is part of the original hypodigm upon which the genus Nexuotapirus was based. The biochronology of this taxon, known elsewhere during the Ar3 to He1, is therefore consistent with the overall age of the Maysville L.F.
Order Artiodactyla Owen, Reference Owen1848
Family Tayassuidae Palmer, Reference Palmer1897
Subfamily Hesperhyinae Prothero, Reference Prothero2015
Hesperhyine tayassuid incertae sedis
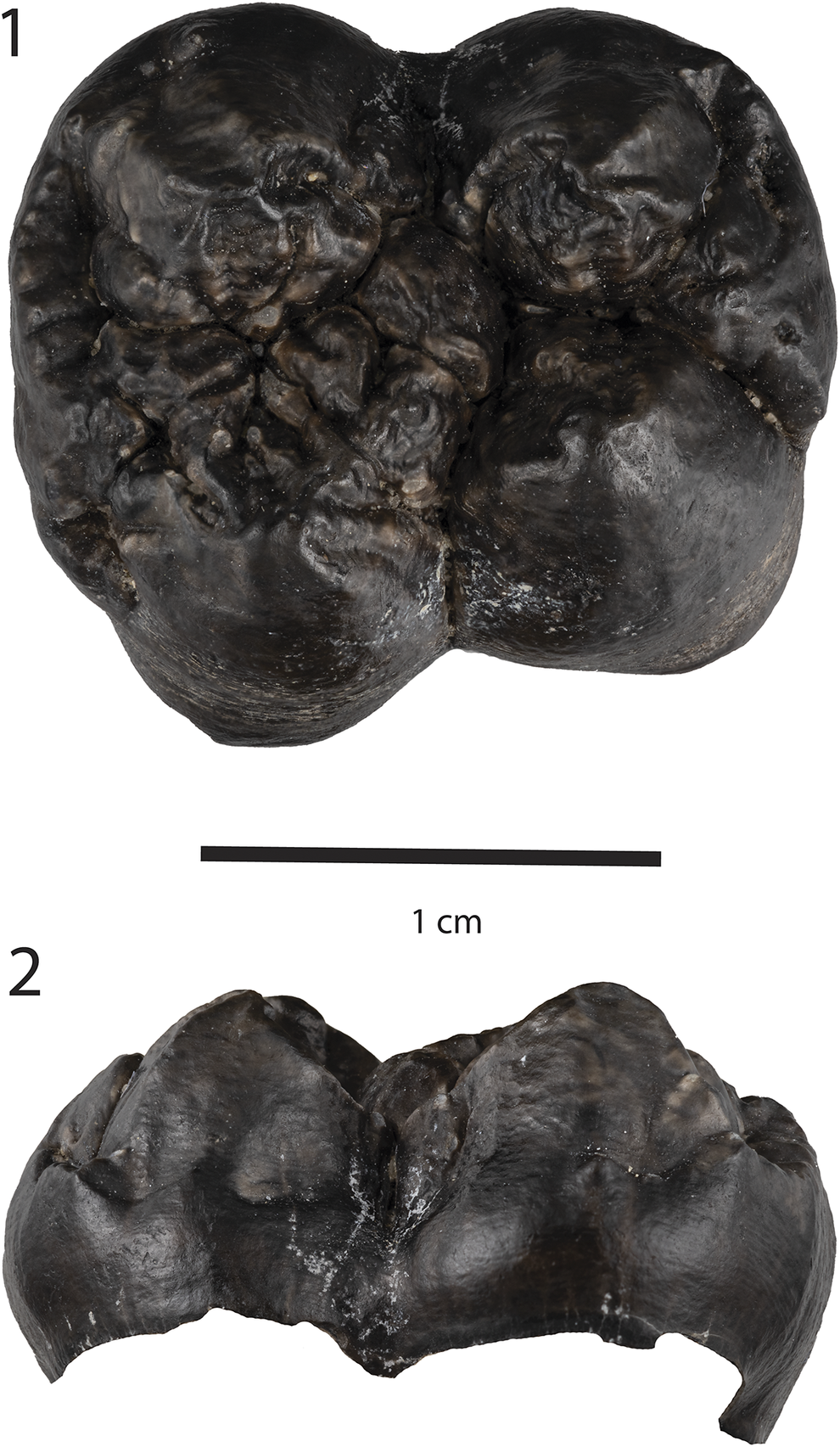
Figure 7. Hesperhyine (Tayassuidae) from the Maysville L.F., USNM 639770, left lower m1 or m2 (1) occlusal, (2) buccal.

Figure 8. Artiodactyla from the Maysville L.F.: Peccary (Tayassuidae), USNM 639776, ?right upper canine (1) ?buccal, (2) ?lingual; (3) UF 552613, left astragalus, dorsal; USNM 639771, Family indeterminate, heavily water-worn astragalus, (4) dorsal.
Occurrence
According to Prothero (Reference Prothero2015), representatives of the subfamily Hesperhyinae that can be referred to the peccary at Belgrade range from the late Arikareean (Ar3) through the Hemingfordian (He2) and are widespread in low to middle latitudes of eastern, middle, and western North America (https://paleobiodb.org).
Description
This lower tooth has the characteristic peccary dental pattern consisting of four principal bunodont cusps of roughly equal height (Fig. 7). These include the anterolingual protoconid, anterobuccal paraconid in the trigonid, and posterolingual entoconid and posterobuccal hypoconid in the talonid. A hypoconulid is well developed in the posterior portion of the talonid. The tooth is four-rooted. The trigonid and talonid are demarcated by a transverse median valley. The trigonid is transversely wider than the talonid. Cingulids are well developed along the anterior and posterior margins of the tooth, and both contain a prominent cuspid. A lingual cingulid is less well developed and the buccal cingulid is absent. A prominent cristid runs posterolingually from the paraconid and terminates in the median valley. Much of the occlusal surface is highly crenulated and has numerous accessory cuspids and stylids. This is much more crenulated than comparable lower molars of Floridachoerus from Thomas Farm, Florida.
The overall size of USNM 639770 (Table 2) is similar to measurements reported for samples of other hesperhyines, including Desmathyus pinensis (Prothero, Reference Prothero2015, table 4). This tooth is significantly larger than other, smaller hesperhyines previously referred to “Cynorca” (e.g., C. proterva from Panama; MacFadden et al., Reference MacFadden, Kirby, Rincon, Montes and Moron2010), although Prothero (Reference Prothero2015, Reference Prothero2021) has revised the concept of this genus considerably.
Materials
USNM 639770, left m1 or m2, collected by Donald Rideout.
Previous reference
Peccary? Lower canine, Bruff et al. (Reference Bruff, Bain, Chandler and Chandler2017, p. 208).
Remarks
Most of the morphological characters used to diagnose Early Miocene peccaries are based on cranial and mandibular characters and, to a lesser extent, on dentitions (Wright, Reference Wright, Janis, Scott and Jacobs1998; Prothero, Reference Prothero2015, Reference Prothero2021). USNM 639770 has been identified as a left m1, but it also could be a m2, because m1s of peccaries are characteristically highly worn; in this case the unworn dental pattern of USNM 639770 is anomalous.
Likewise, individual dental patterns within a given taxon are highly variable (Prothero, Reference Prothero2021) and as such, it is a challenge to definitively allocate a single tooth to a particular genus and species. For the present time USNM 639770 is best referred to as a hesperhyine tayassuid (Prothero, Reference Prothero2015; also see Hesperhyus–“Cynorca” sociale clade, sensu Wright, Reference Wright, Janis, Scott and Jacobs1998) and likely indicates a late Arikareean through Hemingfordian biochron.
Family Tayassuidae Palmer, Reference Palmer1897
Subfamily incertae sedis
Materials
USNM 639776, partial ?right upper canine, collected by Julie Niederkorn; Cary, NC (NC Fossil Club); UF 552613, left astragalus, collected by V. Perez (author).
Previous references
UF 552613, peccary, MacFadden et al. (Reference MacFadden, Bohaska, McCall, Pirlo and Niederkorn2017); Bruff et al. (Reference Bruff, Bain, Chandler and Chandler2017, p. 208).
Remarks
Two specimens from Belgrade represent peccaries but lack sufficient diagnostic characters to be allocated to a more specific taxonomic category. The partial right upper canine, (Fig. 8.1, 8.2) is laterally compressed and has an anterior wear facet for occlusion with the lower canine, which is diagnostic of tayassuids. Bruff et al. (Reference Bruff, Bain, Chandler and Chandler2017, p. 208) described a “peccary? Lower canine” from Belgrade. Their photo represents USNM 639774.
The second specimen, a left astragalus, is characterized by the double pulley shape diagnostic of artiodactyls. It is of medium size relative to other artiodactyls, and the dorsolateral trochlear ridge (Fig. 8.3) is slightly longer than the medial trochlear ridge. Relative to known Early Miocene artiodactyls, based on general size and overall morphology, UF 552613 is most likely a peccary. This specimen lacks the extreme asymmetry and dissimilar parallel trochlear ridge heights seen in other medium-sized artiodactyls (such as oreodonts, not reported from MMBQ), yet these trochlear ridges that are not quite as symmetrical as other contemporaneous medium-sized artiodactyls such as protoceratids that have been reported from the Maysville L.F.
Although perhaps less likely, these two specimens may also represent tayassuids from Plio-Pleistocene deposits (i.e., Duplin Formation) up section, from which fossil land mammals are poorly known (see Appendix).
Family Anthracotheriidae Leidy, Reference Leidy1869
Genus Arretotherium Douglass, Reference Douglass1901
Type species
Arretotherium acridens Douglass, Reference Douglass1901.
Arretotherium cf. A. fricki Macdonald and Schultz, Reference Macdonald and Schultz1956
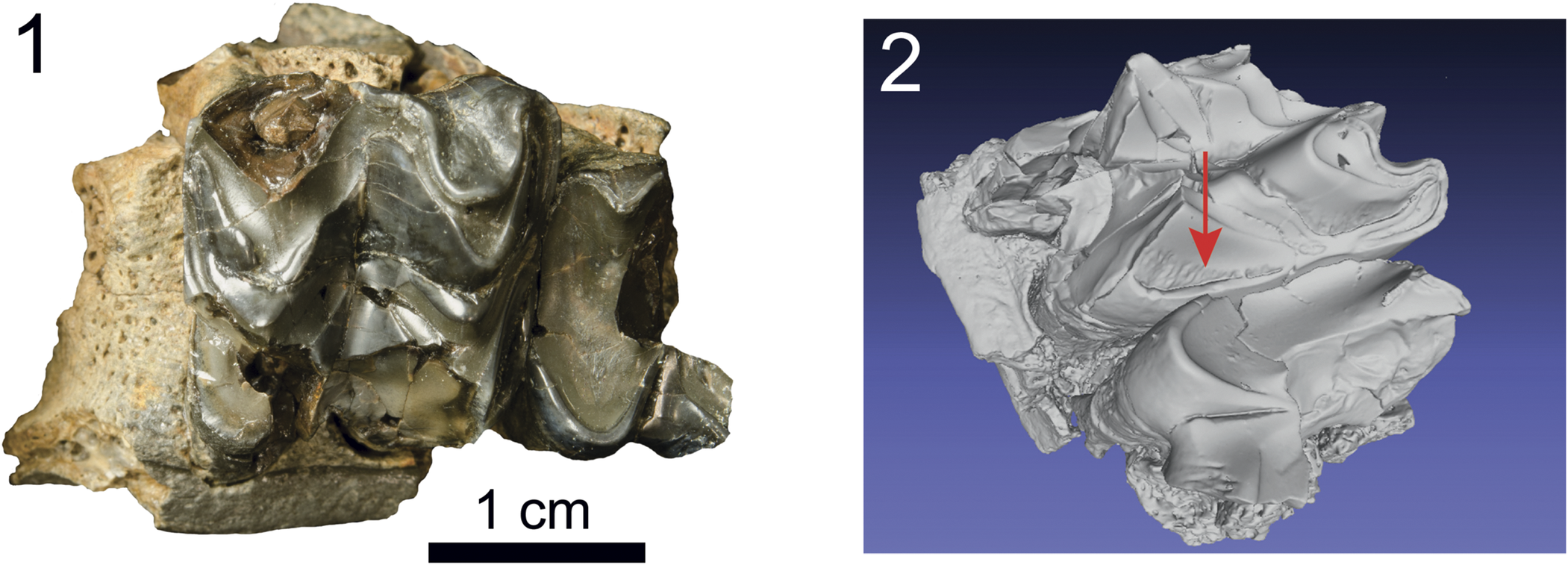
Figure 9. Arretotherium cf. A. fricki (Anthracotheriidae) from the Maysville L.F., UF 552612: (1) maxillary fragment with right M1 (partial) and M2, (2) oblique view showing crenulations on the anterior enamel surface of M2 (arrow).
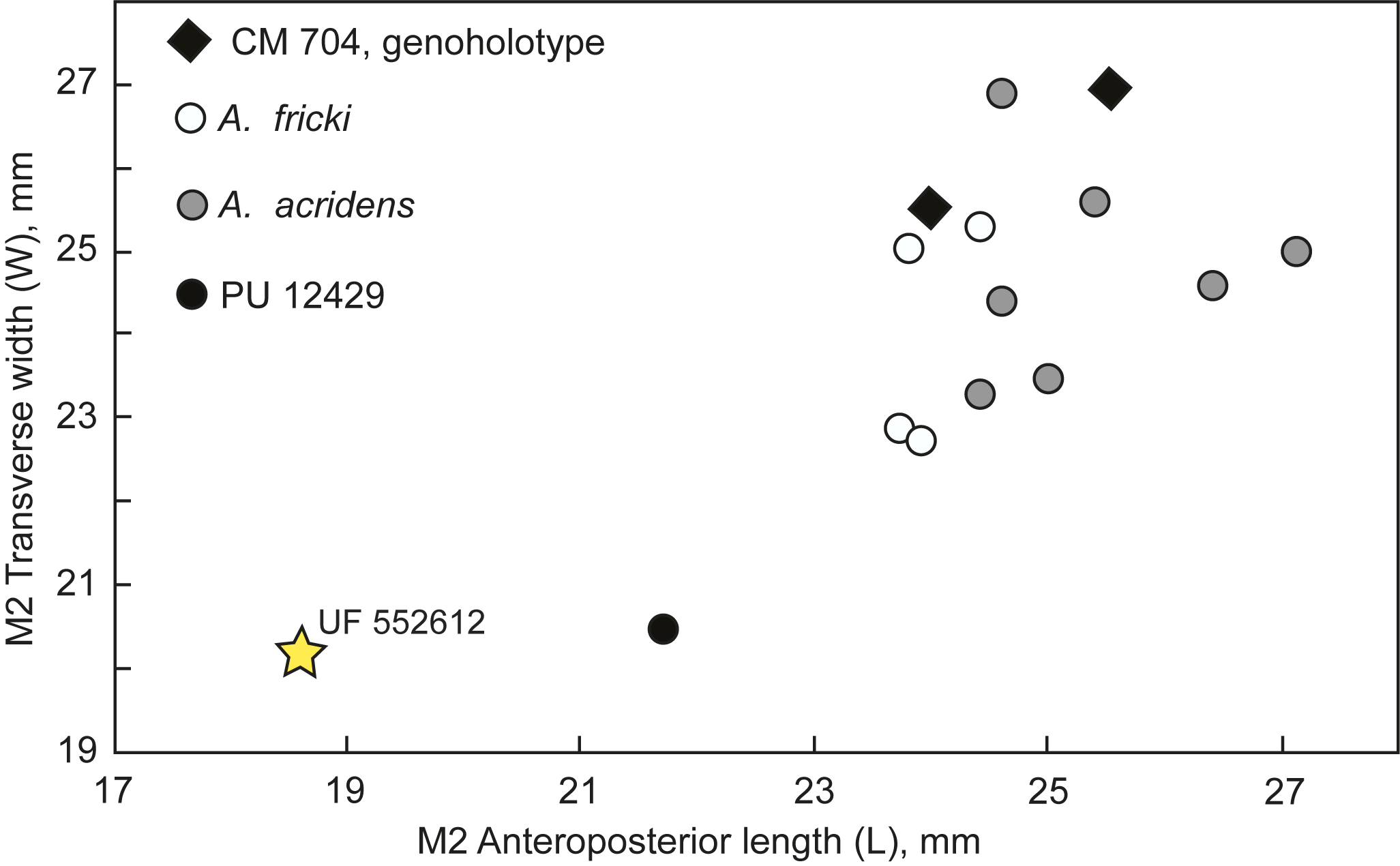
Figure 10. Bivariate plot (L versus W) of M2s of Arretotherium species compared with UF 552612 from the Maysville L.F. (MMBQ). Compiled from Douglass (Reference Douglass1901, p. 277), Macdonald (Reference Macdonald1956, p. 641), and Albright (Reference Albright1998, table 7A). CM 704 is the genoholotype of Arretotherium Douglass, Reference Douglass1901.
Holotype
Badly crushed cranium with R p1, M1–M2, partial M3 (University of Nebraska State Museum 5764) from UNSM locality Bx-12, Hemingford Formation, Hemingfordian (Middle Miocene), Box Butte County, Nebraska.
Occurrence
Early Hemingfordian (He1) of Nebraska, South Dakota, and Saskatchewan (Kron and Manning, Reference Kron, Manning, Janis, Scott and Jacobs1998; Tedford et al., Reference Tedford, Albright, Barnosky, Ferrusquia-Villafranca, Hunt, Storer, Swisher, Voorhies, Webb, Whistler and Woodburne2004; https://paleobiodb.org).
Description
Arretotherium has diagnostic dental characters that separate this genus from other more primitive anthracotheres in North America. Of relevance to the upper cheek tooth dentition, the RM2 of UF 552612 (Fig. 9) from MMBQ demonstrates the diagnostic absence in Arretotherium of the paraconule (sometimes also called the protoconule, even in the same paper) on the anterior region of the molars (e.g., Kron and Manning, Reference Kron, Manning, Janis, Scott and Jacobs1998). The M1 preserves the posterior half of the tooth and contains a perimeter of enamel with heavily worn dentine exposed on the remainder of the surface. The lingual side of the tooth has a moderately well-developed cingulum; the posterior half of the occlusal surface is heavily worn, and therefore, typical M1 enamel characters are not preserved in UF 552612.
The M2 is less worn and preserves much of the typical cheek tooth morphology of this taxon. It is roughly quadrate in occlusal surface outline, with L (18. 6 mm) and W (20.2 mm) being similar in dimensions (Table 2). Important qualitative features include, buccally, well-developed and triangular (pyramidal) paracone and metacone (in mid-wear) separated by a deep transverse valley that actually breeches and separates the ectoloph, or “invades” the mesostyle (Macdonald, Reference Macdonald1956; Kron and Manning, Reference Kron, Manning, Janis, Scott and Jacobs1998). The ectoloph is W-shaped with anterior and posterior crescents. An anterior cingulum is well developed buccally. As mentioned above, the protoconule, which is well developed in other anthracotheres, is absent just posterior to the anterior cingulum, in Arretotherium. The protocone and hypocone regions are broken, so the morphology of these two principal cusps is only partially represented, such as the buccal enamel on the protocone.
In general, UF 552612 is morphologically similar to, although smaller than, the type of A. acridens (Fig. 10), as well as to the upper molars referred to A. acridens from Texas (Albright, Reference Albright1999), A. fricki from Nebraska (Macdonald, Reference Macdonald1956), and A. meridionale Rincon et al., Reference Rincon, Bloch, MacFadden and Jaramillo2013,from Panama (Rincon et al., Reference Rincon, Bloch, MacFadden and Jaramillo2013; also see discussion of Arretotherium from Mexico below). Comparisons with described specimens of Arretotherium from Texas, Mexico, and Panama indicate teeth with highly crenulated enamel surfaces. In Figure 9.2 (arrow), closer inspection in oblique view indicates crenulations at the bottom of the occlusal enamel, suggesting that the crenulations in the other occlusal surfaces were worn down during ontogeny. The presence of crenulations therefore may be characteristic of Arretotherium, particularly for specimens in early wear.
Materials
UF 552612, partial RM1 (posterior half) and RM2 (nearly complete), 3D printed cast of original, also high-resolution digital replica (000604806) available at Morphosource (https://www.morphosource.org). Original collected by Bradley Dixon.
Previous reference
Anthracothere, MacFadden et al. (Reference MacFadden, Bohaska, McCall, Pirlo and Niederkorn2017).
Remarks
Arretotherium acridens and A. fricki are the two dominant species of Arretotherium traditionally recognized in the literature. A third species, A. leptodus (Matthew, Reference Matthew1909), may be distinct, although this is not clear at present (Albright, Reference Albright1999). As mentioned above, Rincon et al. (Reference Rincon, Bloch, MacFadden and Jaramillo2013) described another new species, A. meridionale, from Panama, but this is based primarily on lower dentitions, so meaningful quantitative comparisons of M2s are not available here. Rincon et al. (Reference Rincon, Bloch, MacFadden and Jaramillo2013) indicated a similarity to A. acridens from Texas. In another southern occurrence, Jiménez-Hidalgo and Carbot-Chanona (Reference Jiménez-Hidalgo and Carbot-Chanona2023) described a poorly preserved specimen of Arretotherium from Mexico, but it is not assigned to species. Because of greater size for A. meridionale, uncertainty of A. leptodus, and lack of species designation for Arretotherium from Mexico, these occurrences are not considered further here or in Figure 10.
Focusing on A. acridens and A. fricki, these species apparently represent a chronoclinally evolving series, with the larger A. acridens (and ?A. meridionale) in the late Arikareean and a smaller A. fricki in the Hemingfordian (Macdonald, Reference Macdonald1956; Albright, Reference Albright1998; Tedford et al., Reference Tedford, Albright, Barnosky, Ferrusquia-Villafranca, Hunt, Storer, Swisher, Voorhies, Webb, Whistler and Woodburne2004; Rincon et al., Reference Rincon, Bloch, MacFadden and Jaramillo2013). With one exception (PU 12429, also see Albright, Reference Albright1999), Arretotherium from MMBQ is a distinct outlier at the extreme lower end of the size range for M2s available for this genus (Fig. 10). It likely falls into this chronocline closest to A. fricki, although the size comparison is indeed considerable. As such, the biochronology of UF 552612 indicates an early Hemingfordian (He1) NALMA (Albright, Reference Albright1998; Tedford et al., Reference Tedford, Albright, Barnosky, Ferrusquia-Villafranca, Hunt, Storer, Swisher, Voorhies, Webb, Whistler and Woodburne2004), and is therefore consistent with the observed ranges for other taxa in the Maysville L.F.
Family Entelodontidae Lydekker, Reference Lydekker1883
Genus Daeodon Cope, Reference Cope1878
Type species
Daeodon shoshonensis Cope, Reference Cope1878.
Daeodon shoshonensis Cope, Reference Cope1878

Figure 11. Daeodon shoshonensis (Entelodontidae) from the Maysville L.F., USNM 521238, left m3 (1) occlusal, (2) buccal.
Holotype
Partial symphysis with alveoli, roots, and partial crowns (AMNH 7387), from the John Day Basin, Oregon (Lucas et al., Reference Lucas, Emry and Foss1998).
Occurrence
Early Arikareean (late Oligocene) through middle Hemingfordian (Early Miocene, end of He1; Effinger, Reference Effinger, Janis, Gunnell and Uhen1998; Lucas et al., Reference Lucas, Emry and Foss1998). Effinger (Reference Effinger, Janis, Gunnell and Uhen1998, range chart p. 379) indicated that Dinohyus (Daeodon here) is questionably known from the late Hemingfordian, although it does not occur from well-sampled localities of this age (e.g., Thomas Farm; Hulbert, Reference Hulbert2009). Although never particularly abundant at any given fossil locality, except perhaps for the Agate Springs L.F. in western Nebraska, Daeodon has been found from Oregon, California, Wyoming, South Dakota, a second locality in Nebraska, Texas, Alabama, Florida, South Carolina, and New Jersey (Lucas et al., Reference Lucas, Emry and Foss1998).
Description
USNM 521238 (Fig. 11) represents a left m3 crown (with basal parts of roots missing) in early wear, preserving significant morphological details. Occlusally, the tooth is subquadrate with the talonid being transversely wider (35.5 mm) than the talonid (32.3 mm). The four principal cusps are bunodont, with (1) the trigonid consisting of an anterobuccal protoconid and anterolingual metaconid separated by an anteroposterior valley, and (2) the talonid consisting of a posterobuccal hypoconid and posterolingual entoconid, also separated by an anteroposterior valley. The protoconid and metaconid are taller than either the hypoconid or entoconid. The protoconid and metaconid show prominent anterior and posterior wear facets; the hypoconid and entoconid show prominent anterior facets, in the posterior portion of these latter two cusps wear facets are not developed. The transverse valley separating the trigonid and talonid that is characteristic of bunodont artiodactyls is deep and well developed. Near the center of this valley along the buccal side of the entoconid, accessory cuspids are present, with the larger one being anterobuccal and another being posterobuccal in position. Likewise, a smaller but distinct accessory stylid, probably the hypostylid, arises from the anterior buccal region of the hypoconid.
Two tiny, rudimentary stylids are poorly developed on the anterobuccal side of the tooth in the position where a cingulum, although absent, would be. Diagnostic of the lower m3 posterior talonid, a hypoconulid is well developed posteriorly and consists of a primary cuspid just lingual to the anteroposterior midline flanked along a well-developed posterior cingulum by one smaller stylid lingually and three stylids buccally. Whereas this hypoconulid region has highly crenulated (rugose in Lucas et al., Reference Lucas, Emry and Foss1998) enamel in the occlusal and lateral surfaces of this region, elsewhere on this tooth the enamel is either smooth (e.g., on the wear facets described above) or only slightly crenulated.
Materials
USNM 521238, left m3, collected by Bonnie McDaniel.
Previous references
Daeodon sp. Bruff et al. (Reference Bruff, Bain, Chandler and Chandler2017, p. 165); entelodont (Daeodon), MacFadden et al. (Reference MacFadden, Bohaska, McCall, Pirlo and Niederkorn2017).
Remarks
The overall set of characters described above for dentition USNM 521238 are diagnostic of Daeodon, and if only one species is considered valid (Lucas et al., Reference Lucas, Emry and Foss1998), then it pertains to D. shoshonensis. The size of USNM 521238 falls within the range, although at the smaller end, of tooth length and width measurements reported for this species (Lucas et al., Reference Lucas, Emry and Foss1998, table 1). USNM 521238 is very similar to the description of YPM 12041, also a left m3. Primary differences include: (1) YPM 12041 has well-developed buccal and lingual cingulids, whereas these are lacking in USNM 521238; and (2) the accessory cuspids along the buccal side of the entoconid and accessory stylids flanking the posterior hypoconulid in USNM 521238 are not apparent in the illustration of YPM 12041 (Lucas et al., Reference Lucas, Emry and Foss1998, fig. 3D and E). The observed differences between these two specimens are regarded as individual intraspecific variation.
Bruff et al. (Reference Bruff, Bain, Chandler and Chandler2017, p. 165) described two relevant Daeodon specimens. Their USNM 521238, listed as “Ammodon leidyanus” Marsh, Reference Marsh1893, from Onslow County, is the same molar that we describe above. The other specimen illustrated on page 165 is also listed from Onslow County, but was not available for examination during this study. Although indicated as coming from Onslow County, these likely come from MMBQ in Jones County.
Family Protoceratidae Marsh, Reference Marsh1891
Genus Paratoceras Frick, Reference Frick1937
Type species
Paratoceras macadamsi Frick, Reference Frick1937.
Paratoceras wardi Patton and Taylor, Reference Patton and Taylor1973
Figures 12, 13, Tables 1, 2
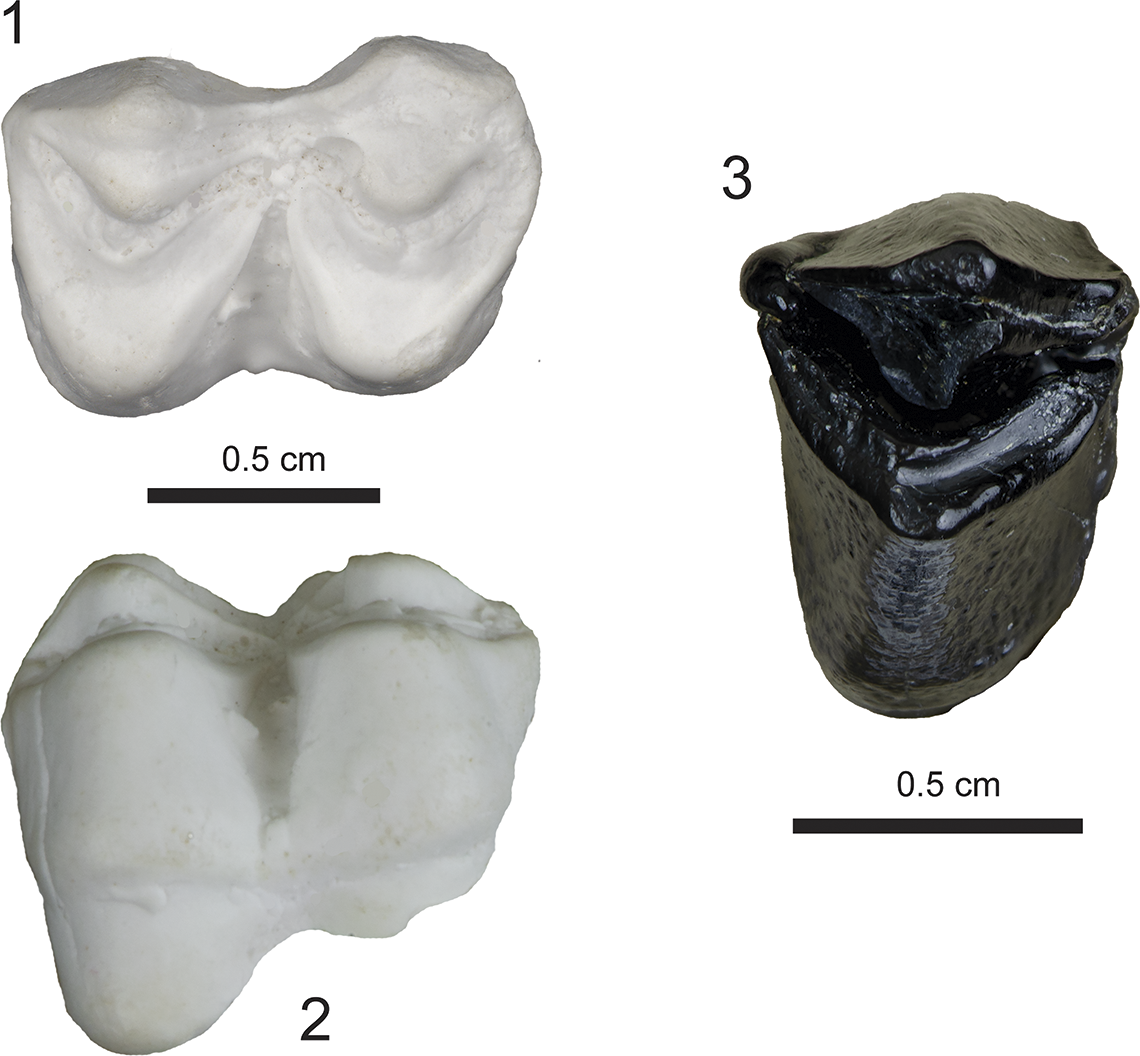
Figure 12. Specimens referred to Paratoceras wardi (Protoceratidae) from the Maysville L.F., USNM 639766, right m1 (plaster cast), (1) occlusal, (2) buccal; UF 553040, partial R lower molar (posterior half) (3) occlusal.
Holotype
Nearly complete skull with left orbital horn, occipital horn, and C (alveolus)–M3 (F:AM [Frick:American Mammals] 40249), from the early Barstovian (Ba1) Trinity River Pit 1, San Jacinto County, Texas (Patton and Taylor, Reference Patton and Taylor1973).
Diagnosis
The m1 anteroposterior length of Paratoceras wardi is larger than P. orarius Rincon et al., Reference Rincon, Bloch, MacFadden and Jaramillo2015, and smaller in both m1 anteroposterior length and m1 transverse width than P. coatsi Rincon et al., Reference Rincon, Bloch, MacFadden and Jaramillo2015 (Fig. 13), or P. macadamsi (Patton and Taylor, Reference Patton and Taylor1973).
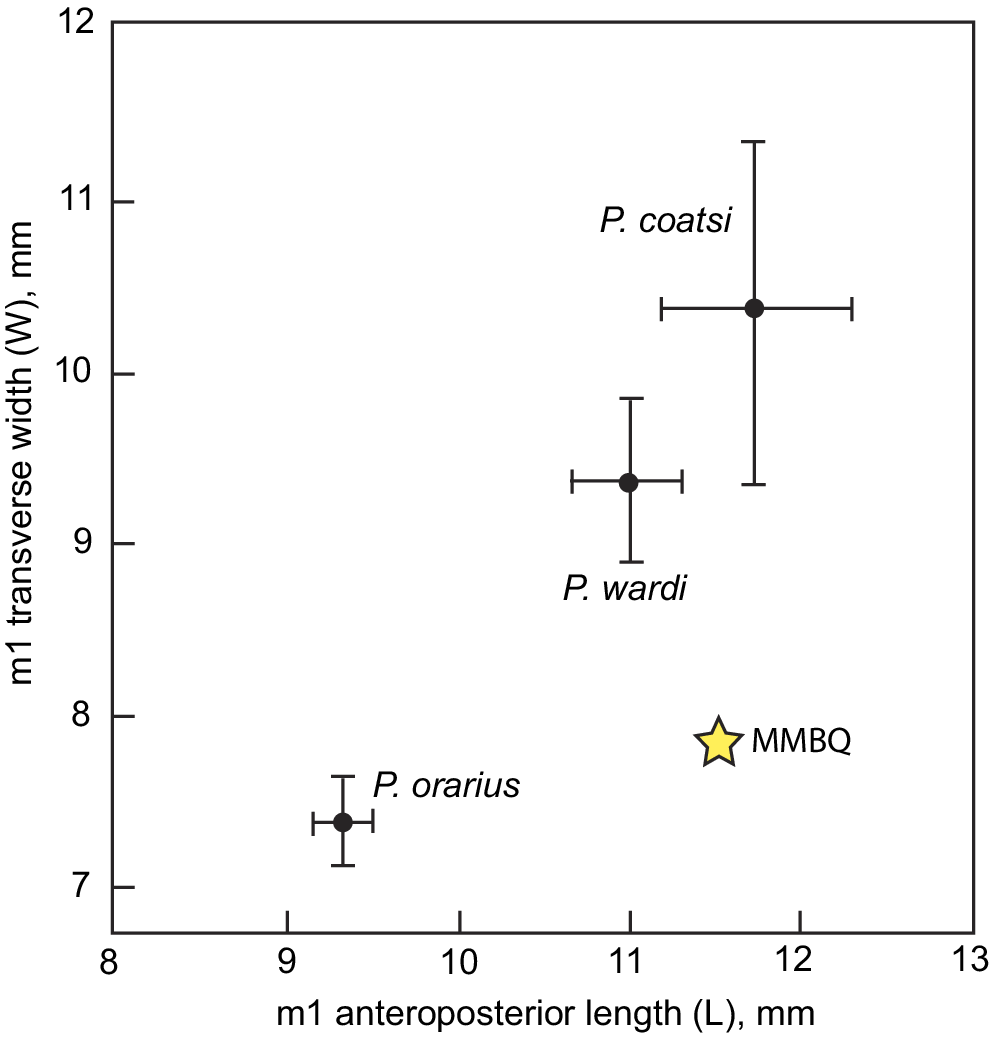
Figure 13. Bivariate plot (L versus W) of m1s of relevant Paratoceras species samples (Rincon et al., Reference Rincon, Bloch, MacFadden and Jaramillo2013) compared with UF 552612 from the Maysville L.F from MMBQ (yellow star).
Occurrence
Paratoceras wardi spans from the latest Arikareean (Ar4) to early Barstovian (Ba1) in southeastern North America, including Panama (MacFadden, Reference MacFadden2006; Rincon et al., Reference Rincon, Bloch, MacFadden and Jaramillo2013).
Description
USNM 639766 preserves a moderately worn right m1 (Fig. 12.1, 12.2). It has characteristic protoceratid morphology, including a selenodont dentition with a continuous anteroposterior lophid. Transversely, crescents of similar size are developed with an anterior protolophid (protoconid) and posterior hypolophid (hypoconid). A faint cingulid is developed on the protoconid; and this is even fainter on the hypoconid. An intercolumnar pillar (Rincon et al., Reference Rincon, Bloch, MacFadden and Jaramillo2013) is faintly developed in the buccal side of the tooth between the protolophid and hypolophid. The occlusal surface of the tooth measures L = 11.5 mm; W = 7.8 mm. This specimen compares favorably in dental pattern, size, and stage of wear with those referred to P. wardi (USNM 23154, 23155) from the Centenario Fauna of Panama (MacFadden et al., Reference MacFadden, Bloch, Evans, Foster, Morgan, Rincon and Wood2014), as well as from the type hypodigm from Texas (Patton and Taylor, Reference Patton and Taylor1973).
UF 553040 (Fig. 12.3) preserves a single lophid, with a possible broken connection anteriorly, suggesting that it may be the heel of a m3. While the exact position, and even whether this is a lower or upper molar fragment is debatable, the development of the style and ribs on the lingual surface suggests more that this is a partial lower tooth.
Materials
USNM 639766 tooth (plaster cast) with R m1, collected by Mickey Monroe; UF 553040, partial R lower molar (posterior half), collected by Diane Willis (NC Fossil Club) in situ from the sequestered Belgrade Formation piles on May 24, 2019.
Previous reference
Small selenodont artiodactyl (?protoceratid), MacFadden et al. (Reference MacFadden, Bohaska, McCall, Pirlo and Niederkorn2017).
Remarks
Although protoceratids were originally distinguished primarily by their cranial appendages (Frick, Reference Frick1937), assuming sufficient preservation of dentitions, the described species of Paratoceras can be recognized based on size of their dentition, including measurements of their teeth and selected ratios. Prior to this paper, five species of Paratoceras had been described in the literature: P. wardi and P. macadamsi, both from Texas (Patton and Taylor, Reference Patton and Taylor1973), P. tedfordi Webb et al., Reference Webb, Beatty and Poinar2003, from Mexico, and two species, P. orarius and P. coatsi, from Panama. Patton and Taylor (Reference Patton and Taylor1973) reviewed P. wardi and P. macadamsi, and MacFadden (Reference MacFadden2006) described P. wardi from Panama.
Both P. macadamsi (MacFadden, Reference MacFadden2006) and P. tedfordi (20% larger than P. wardi, according to Webb et al., Reference Webb, Beatty and Poinar2003) are too large to pertain to the protoceratid species from MMBQ (Fig. 13). Of relevance here, MacFadden (Reference MacFadden2006) recognized P. wardi from Early Miocene sediments cropping out along the Panama Canal. Rincon et al. (Reference Rincon, Bloch, MacFadden and Jaramillo2013) then describe two new species from the same sequence in Panama, a smaller P. orarius and a larger P. coatsi, the latter of which they indicated to be a better name, distinct from P. wardi (MacFadden, Reference MacFadden2006).
In the interpretation here, P. wardi is metrically distinct (Fig. 12) from P. coatsi and P. orarius. The single complete lower tooth of Paratoceras from the MMBQ is closest to P. wardi from Panama (Fig. 13), and therefore this nomen is used here. While it is a challenge to diagnose and allocate protoceratids on a single tooth that is in common with the various samples described above, it can logically be argued (Fig. 13) that P. wardi occurs at the MMBQ.
The presence of the protoceratid Paratoceras wardi from Belgrade is the northernmost occurrence of this species, which otherwise extends into Texas (Patton and Taylor, Reference Patton and Taylor1973) and Panama (MacFadden, Reference MacFadden2006). The biochron of this species ranges from the late Arikareean in Panama to the early Barstovian in Texas. Given the biochronology of the other index taxa from the Maysville L.F., the occurrence of P. wardi from MMBQ is consistent with the early part of the range for this species being late Arikareean (Ar4) to early Hemingfordian (He1) NALMA within the Runningwater Chronofauna.
Order Artiodactyla Owen, Reference Owen1848
Family incertae sedis
Description
This specimen has the diagnostic artiodactyl double pulley shape (Fig. 8.4) with, in dorsal aspect, parallel medial and lateral trochlear ridges that are subequal in size, and a prominent deep central fossa and groove are well developed between the trochlea. On the plantar side a prominent convex sustentacular facet is well developed laterally. The greatest length from the tip of the lateral trochlea to the base of the distal keel is 23.4 mm; the transverse width across the trochlea is 13.2 mm.
Materials
USNM 639771, water-worn astragalus, collected by Julie Niederkorn; Cary, NC (NC Fossil Club).
Previous reference
Euungulata [sic.], Bruff et al. (Reference Bruff, Bain, Chandler and Chandler2017, p. 207).
Remarks
USNM 639771 is too small to be identified as either a peccary (Table 2) or the protoceratid Paratoceras wardi (two astragali of the latter measured 29 mm in Patton and Taylor, Reference Patton and Taylor1973, p. 387); it also is larger than the tiny tragulid Machaeromeryx from Thomas Farm, Florida. It does, however, compare favorably in size and general morphology with the moschid artiodactyl from the Miller Site in Florida, and with Parablastomeryx floridanus White, Reference White1940, from Thomas Farm. There are, however, many other potential comparisons with other “hornless ruminants” (Webb, Reference Webb, Janis, Scott and Jacobs1998) that existed during the Early Miocene in North America. Given the many other potential options, and the fact that USNM 639771 is heavily water worn, a more definitive taxonomic identification is not warranted at this time. Bruff et al. (Reference Bruff, Bain, Chandler and Chandler2017, p. 207) presented photos of a water-worn astragalus that almost certainly represents USNM 639771 described here.
Results
Biochronology of Maysville L.F. mammals
Taken together, the land mammal taxa that comprise the Maysville L.F. provide a meaningful age interval (biochron) for the Belgrade Formation at MMBQ (Table 2). As indicated in Figure 14, the long-lived beaver cf. Palaeocastor sp. and stem lagomorph cf. Megalagus sp., while not constraining the early part of the biochron, do limit the later range of the Maysville L.F. by not extending past, respectively Ar4 and He2. Another taxon, the entelodont Daeodon shoshonensis, questionably occurs in Ar2. Its definitive first occurrence is in Ar3, and it last occurs in He1. Three taxa first occur in Ar3 (Fig. 14): the tapir Nexuotapirus marslandensis, hesperhyine peccary, and questionably the long-lived horse genus Anchitherium. All three are also known to occur in Ar4. Nexuotapirus marslandensis extends into He1, the hesperhyine peccary through He2, and Anchitherium into Ba1. Paratoceras wardi first occurs in Ar4 and ranges through Ba1. The rhinoceros cf. Menoceras barbouri questionably occurs in the Ar4 and extends through the Hemingfordian (both He1 and He2). There are four remaining biochronologically useful land mammal taxa in the Maysville L.F.: the horse Archaeohippus blackbergi, which occurs in He1 and He 2; whereas the anthracothere Arretotherium cf. A. fricki and carnivorans Craterogale cf. C. simus and Amphictis cf. A. timucua all are restricted, so far as is known elsewhere, to He1.
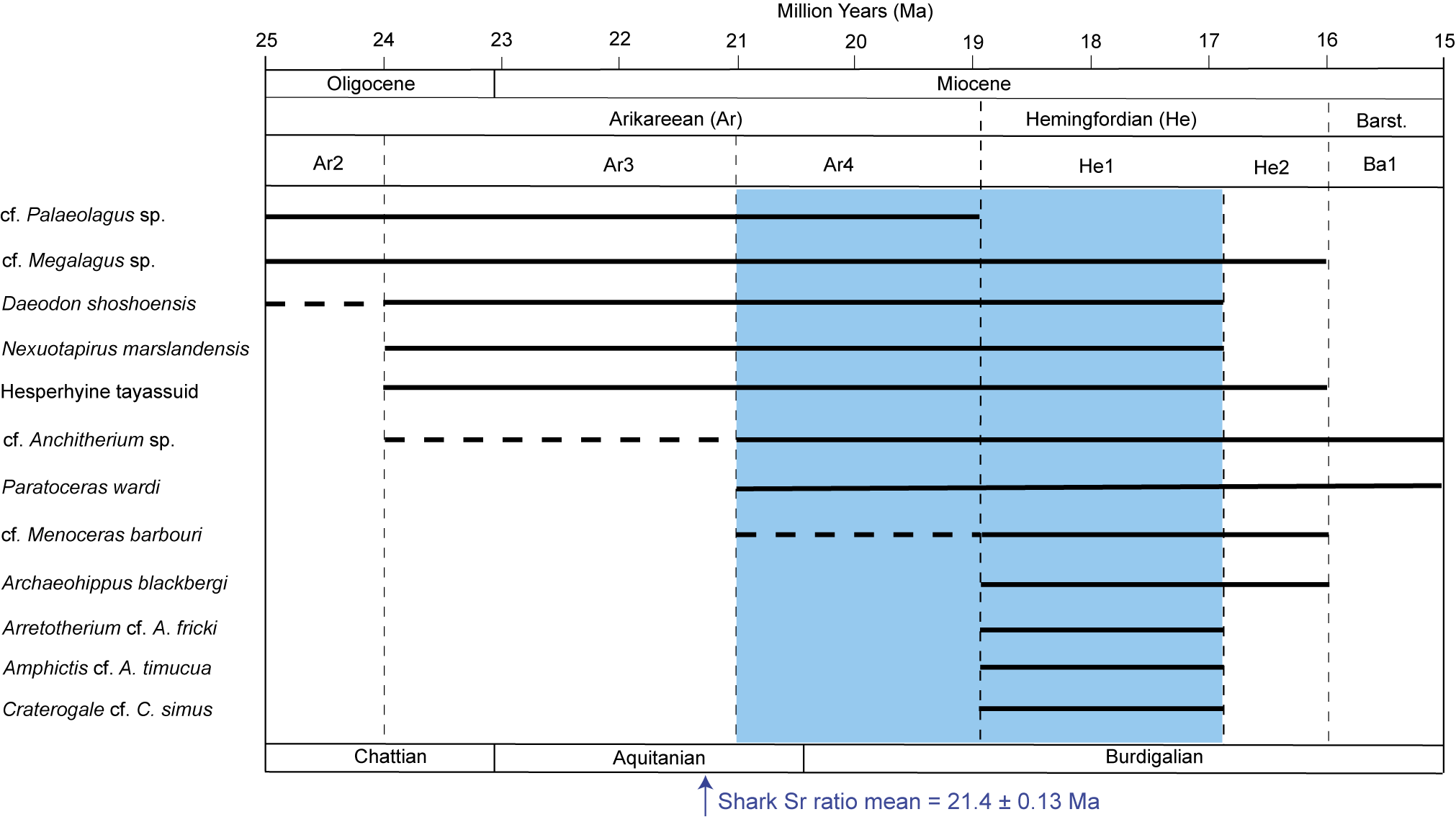
Figure 14. Biochronological ranges for the taxa that inform the age of the Maysville L.F. (also see Table 2), arranged in order of first appearance. Arrow at bottom indicates mean Sr age (see Fig. 15). Cenozoic epoch and marine stage boundaries follow ICS (2023); NALMAs follow Albright (Reference Albright1998) and Tedford et al. (Reference Tedford, Albright, Barnosky, Ferrusquia-Villafranca, Hunt, Storer, Swisher, Voorhies, Webb, Whistler and Woodburne2004). Dashed lines represent questionable occurrences. Barst. = Barstovian.
Following prior recommendations and practice (e.g., Wood et al., Reference Wood, Chaney, Clark, Colbert, Jepsen, Reeside and Stock1941; Tedford, Reference Tedford1970; Woodburne, Reference Woodburne and Woodburne1987; Tedford et al., Reference Tedford, Albright, Barnosky, Ferrusquia-Villafranca, Hunt, Storer, Swisher, Voorhies, Webb, Whistler and Woodburne2004), individual NALMA biochrons are primarily characterized by first appearances, last appearances, unique (index) taxa, and characteristic taxa (that may extend before and/or after inferred NALMA subcrons). In the Tedford et al. (Reference Tedford, Albright, Barnosky, Ferrusquia-Villafranca, Hunt, Storer, Swisher, Voorhies, Webb, Whistler and Woodburne2004), characterization of the Miocene NALMAs, two relevant immigrant taxa that also occur in the Maysville L.F. inform our biochronological analysis described here. The genus Menoceras defines the base of Ar3, although the species M. barbouri questionably first occurs in Ar4 and then definitely occurs throughout the Hemingfordian. Also, according to Tedford et al. (Reference Tedford, Albright, Barnosky, Ferrusquia-Villafranca, Hunt, Storer, Swisher, Voorhies, Webb, Whistler and Woodburne2004), Amphictis defines the Ar4/He1 boundary (Baskin et al., Reference Baskin, Dickinson, DuBois, Galiano and Hartstone-Rose2020 mentioned that ?Amphictis from Belgrade may be Ar4 in age, but this likely was influenced by the early assessment of the age of the Maysville L.F. by MacFadden et al., Reference MacFadden, Bohaska, McCall, Pirlo and Niederkorn2017).
In summary, based only on the biochronology described above, and if Archaeohippus blackbergi, Amphictis cf. A. timucua and Craterogale cf. C. simus represent previously unrecognized, earlier range extensions, then the Maysville L.F. most likely indicates latest Arikareean (Ar4) to early Hemingfordian (He1) NALMAs. This interval has been previously referred to as the Runningwater Chronofauna (e.g., Webb and Opdyke, Reference Webb and Opdyke1995; Janis et al., Reference Janis, Colbert, Coombs, Lambert, MacFadden, Mader, Prothero, Schoch, Shoshani, Wall, Janis, Scott and Jacobs1998) because of similar assemblages of evolving chronospecies during this time. In addition to the native fauna, this also was a time of numerous dispersal events into North America (Qiu, Reference Qiu2003; Tedford et al., Reference Tedford, Albright, Barnosky, Ferrusquia-Villafranca, Hunt, Storer, Swisher, Voorhies, Webb, Whistler and Woodburne2004). These immigrations are likely related to multiple episodes of drops in global sea level, some as much as 150 m lower than present, that potentially opened up land bridges during the Early Miocene between about 23 and 18 Ma (Qiu, Reference Qiu2003; Tedford et al., Reference Tedford, Albright, Barnosky, Ferrusquia-Villafranca, Hunt, Storer, Swisher, Voorhies, Webb, Whistler and Woodburne2004; Haq and Ogg, Reference Haq and Ogg2024). This is a conceptually interesting time interval in Cenozoic land mammal evolution. Likewise, this biochronological assessment will be further informed with the additional geochronological data from Belgrade Formation provided below.
Geochronological analysis: Strontium Isotope Stratigraphy (SIS)
Seven individual shark teeth collected in situ from the Belgrade Formation were sampled three times each. Mean values for the three samples taken from each specimen were again averaged to produce an overall mean age of 21.4 ± 0.13 Ma (Fig. 15). This same protocol was implemented for both the oysters (four individual samples) and venerid clams (four individual samples) from the Belgrade Formation and produced overall mean ages of 28.0 ± 0.22 Ma and 27.6 ± 0.26 Ma, respectively (Supplementary Material 1).
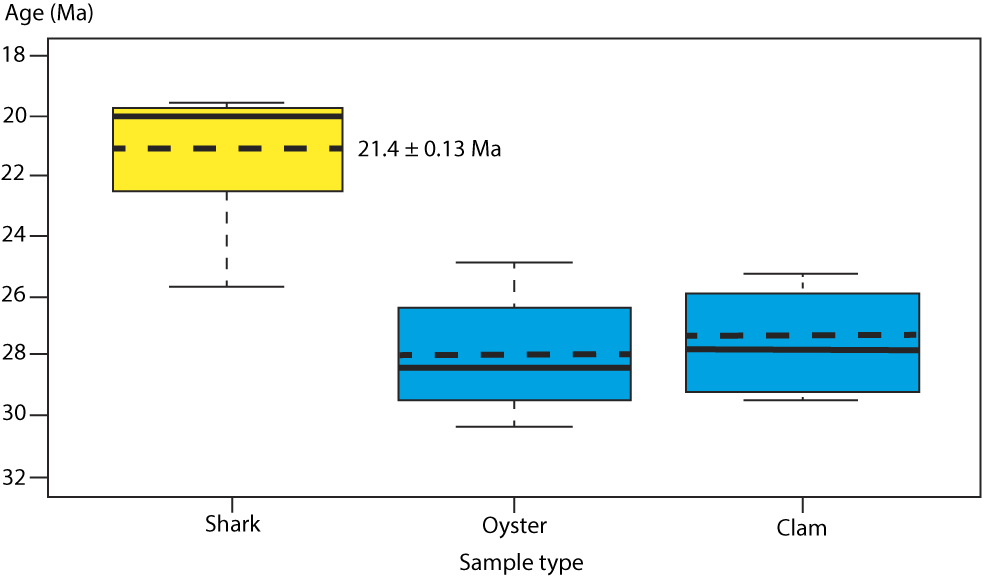
Figure 15. Boxplot of median sample ages for shark (Hemipristis and Carcharias, N = 7), oyster (Striostrea, N = 4), and clam (Chione, N = 4) specimens showing medians (solid line) within the interquartile range (IQR). The dashed lines indicate the mean age based on the shark teeth for the Maysville L.F. compared to those of the oyster (28.0 ± 0.22 Ma) and clam (27.6 ± 0.26 Ma). The original data, statistics, and R code are presented in Supplemental Materials at https://doi.org/10.5061/dryad.7h44j103k.
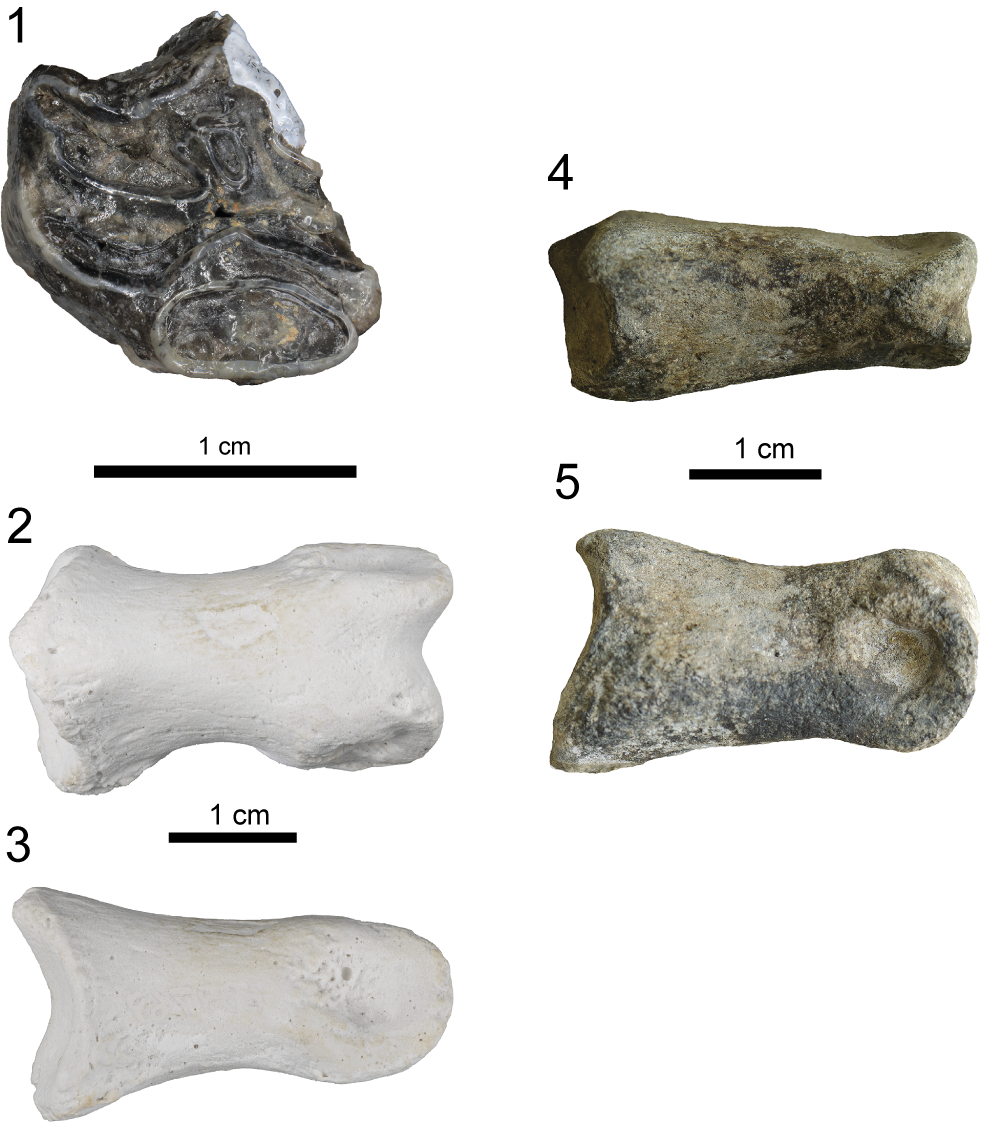
Figure 16. Plio-Pleistocene land mammals from the Martin Marietta Belgrade Mine: (1) Horse Nannippus peninsulatus (Cope, Reference Cope1885), USNM 639777, partial upper cheek tooth in advanced wear stage, occlusal; Bear (Ursidae), USNM 639769, medial phalanx (2) anterior (dorsal), (3) lateral; Odocoileus virginianus (Zimmermann, Reference Zimmermann1780) (Cervidae), USNM 639775, medial phalanx, (4) anterior (dorsal), (5) lateral.
The median and mean ages for sharks, oysters, and venerid clams are depicted in Figure 15. A Shapiro–Wilk test for normality that was completed using those mean ages yielded p-values of 0.06335, 0.7178, and 0.5233 respectively, indicating our sample group data are normally distributed. Given this distribution, using one-way ANOVA and Tukey’s HSD (honestly significant difference) tests, multiple comparisons of the means at 95% confidence interval indicate that ages from the shark specimens are statistically distinct from both the oyster and clam ages, the latter two of which are indistinguishable.
Discussion: integrated chronology, stratigraphy and paleoecology of the Belgrade Formation and Local Fauna
Given the results presented here, two lines of evidence (i.e., land mammal biochronology and SIS) inform the age interpretation for the Belgrade Formation at MMBQ in Jones County, North Carolina. The most inclusive interpretation regarding the land mammals (Fig. 14) indicates a late Arikareean (Ar3/Ar4) through late Hemingfordian (He2) age, or, using the calibrations of Tedford et al. (Reference Tedford, Albright, Barnosky, Ferrusquia-Villafranca, Hunt, Storer, Swisher, Voorhies, Webb, Whistler and Woodburne2004), an interval from about 24–16 Ma. We assert, however, that the strongest land mammal age signal is from the Ar4 through He1, or an interval of 21–17 Ma (Fig. 14).
The SIS of the invertebrates from MMBQ not only corroborate the land mammal age calibrations, but also address differing interpretations about the relative ages of the Pollocksville and Haywood Landing members of the Belgrade Formation, which crops out in section 20 (Fig. 1). A mean age for the sharks (Hemipristis and Carcharias) of 21.4 ± 0.13 Ma (Supplemental Material) is consistent with a late Ar3/Ar4 NALMA for the Maysville L.F. Taxa whose established occurrences extend into the Hemingfordian (Fig. 14), based on evidence elsewhere in North America, thus represent younger portions of known biochronological ranges. On the other hand, the SIS ages (Supplemental Material) of 28.0 ± 0.22 Ma for the oysters (Striostrea) and 27.6 ± 0.26 Ma for the clams (Chione) are statistically indistinguishable, as indicated by Tukey’s HSD multiple comparisons of means between groups. Therefore, these bivalves indicate an age older than that of the Maysville L.F. Given this evidence, the unambiguous interpretation here is that the invertebrates support an older, late Oligocene age and likely come from the Pollocksville Member, whereas the shark teeth and land mammals support a younger, Early Miocene age and likely come from the Haywood Landing Member of the Belgrade Formation, which crops out in section 20 at MMBQ (Fig. 1). However, Baum et al. (Reference Baum, Harris and Zullo1978) interpreted the Crassostrea (i.e., Striostrea) beds to have been deposited by later channel downcutting into the Belgrade Formation. If this is the case, then our SIS ages reported here may be anomalous because the original Sr isotopic values could have been changed by freshwater inputs. However, this seems unlikely given the consistency in Sr values produced by both Striostrea and Chione.
An informed discussion of the Maysville L.F. paleoecology has been hampered in the past because of the: (1) lack of definitively associated, in situ vertebrate faunal elements; and (2) uncertain stratigraphic interpretations of the correspondence between the Pollocksville and Haywood Landing members of the Belgrade Formation. Given our study here, however, we can now elucidate the paleoecology of the Maysville L.F. as represented by the specimens presumed, or known, to be derived from the Haywood Landing Member. This assemblage is generally similar in its paleoecology to many Neogene faunas in eastern North America with taphonomically mixed marine and non-marine components (Tedford and Hunter, Reference Tedford and Hunter1984). These represent faunas preserved in nearshore coastal, estuarine, and typically low-elevation terrestrial and possibly riparian environments. For example, the herbivores preserved from Maysville L.F. are characterized primarily by browsers, lacking any higher-crowned taxa trending towards grazing (e.g., Parahippus leonensis Sellards, Reference Sellards1916), otherwise known from He1 sites elsewhere in North America during this time. Thus, the terrestrial mammals described here include species that would have lived in primarily open country, riparian, and woodland habitats, but lacking dense forests or extensive grasslands. The marine vertebrate species from the Maysville L.F., although not described in this paper, include chondrichthyans, osteichthyans, cetaceans, sirenians, chelonians, and crocodilians. The abundant and diverse chondrichthyan fauna (Wilson et al., Reference Wilson, Perez, Magallanes and MacFadden2019), including taxa such as Carcharhinus, Rhizoprionodon, Negaprion, Hemipristis, Ginglymostoma, and Anoxypristis, represent a nearshore paleoenvironment with a water depth of less than 200 m.
Concluding comments and significance
Given the paucity of Early Miocene land mammal assemblages in eastern North America, the Maysville L.F. at MMBQ fills an important gap in our knowledge of this time interval and paleogeographic region. It also further illustrates the concept of the Runningwater Chronofauna (e.g., Webb and Opdyke, Reference Webb and Opdyke1995), a time interval that started with several pulses of Eurasian immigrants during lowered global sea level, including the rhinoceros Menoceras during the late Arikareean and carnivores Amphictis and Craterogale at the beginning of the Hemingfordian, all of which are known from MMBQ. These immigration events occurred during an interval within the late Arikareean through the Hemingfordian in which for at least 6 Myr, land mammal assemblages maintained much of their chronofaunal integrity. It is therefore very difficult to differentiate, for example, between an Ar4 and a He1 local fauna.
The Maysville L.F. contains not just land mammals, but also marine vertebrates, including abundant and diverse chondrichthyans (Wilson et al., Reference Wilson, Perez, Magallanes and MacFadden2019), bony fishes, reptiles, marine mammals (Boessenecker, Reference Boessenecker2022), and invertebrates. In contrast, many of the well-known Early Miocene land mammal sites in eastern North America, including Thomas Farm, Florida, are sinkhole deposits that lack marine components. Fossiliferous sites such as MMBQ with marine and non-marine tie-ins are thus exceedingly valuable for correlations, not just for NALMAs but for correspondence with marine stages (Tedford and Hunter, Reference Tedford and Hunter1984). Likewise, we can now test biochronological faunal assessments with age-dependent Sr isotope variations in ancient seawater. In this particular case, SIS essentially overlaps with the age of the late Arikareean (AR3/Ar4) land mammals at 21.4 ± 0.13 Ma and resolves a controversy with demonstrably older late Oligocene invertebrates. This integrated approach using paleontology and geochemistry thus further refines Miocene faunal calibrations and fills an important geographic and temporal gaps in eastern North America.
Data availability statement
The data used or produced in this report are available as follows: (1) fossil specimens; all the specimens described in this paper are vouchered in the vertebrate paleontology research collections primarily at the NCSM, NMNH, and UF; aggregated sets of these specimen collections data are available at iDigBio and GBIF; (2) analytical data and measurements taken on fossil specimens are included in the text or Table 2. Geochemical data and related statistical tests used for the SIS analyses are included in Supplementary Materials at https://doi.org/10.5061/dryad.7h44j103k.
Acknowledgments
We are grateful to Martin Marietta for access to MMBQ and D. Fetsko, Mine Manager, and D. Humphrey, for access to the quarry to collect fossils. We also appreciate their extra efforts to excavate piles of Belgrade Formation in situ sediments for our use in this research. We thank the many citizen scientists who, over the past few decades, have enthusiastically collected fossils from the Belgrade Mine, many of whom are acknowledged above. J. Niederkorn, who collected and donated most of the specimens described above, deserves special recognition here. This important local fauna would not be as complete without their contributions. We also thank the staff and volunteers from the USNM, including J. Nakano for arranging loans and the paleo laboratory volunteers for their countless hours picking and sorting sedimentary matrix from Belgrade. We thank C. O’Connor, a volunteer in our collection, for sorting the incoming specimens, picking microfaunal matrix, and identifying the fossil sharks, all from Belgrade. We also thank B. Albright, W. Korth, D. Prothero, and A. Woodruff for their help with specimen identifications, Z. Randall for the specimen photographs, and R. Hulbert for general advice. C. Sagebiel advised us on the status of the type of Archaeohippus blackbergi and D. Jones advised us with the SIS dating of the invertebrates. We thank G. Kamenov for his help with the SIS procedures and laboratory analysis using the ICPMS at the University of Florida. Part of this research, particularly during the collecting stage, was supported by the NSF-funded myFOSSIL project (1322725). This is University of Florida Contribution to Paleobiology 892.
Competing interests
The authors declare that there are no competing interests with this research and manuscript.
Appendix. Plio-Pleistocene land mammals from MMBQ, North Carolina
During this study it became clear that at least three land mammal specimens catalogued in the USNM collections as coming from MMBQ cannot be Early Miocene in age (i.e., part of the Maysville L.F.) because of their general morphology and/or stage of evolution. These are most likely derived from post-Miocene sediments that overlie the Belgrade Formation at MMBQ (Fig. 1). Ward et al. (Reference Ward, Lawrence and Blackwelder1978) included these as the Plio-Pleistocene Duplin and Croatan formations capped by “unstudied later Pleistocene deposits” (see Fig. 1).
Nannippus peninsulatus (Equidae), USNM 639777
This partial left upper cheek tooth (Fig. 16.1), consisting of ?P3 or P4, preserves well-worn anterior and lingual portions of the occlusal surface and crown. Bruff et al. (Reference Bruff, Bain, Chandler and Chandler2017, p. 190) previously described and illustrated this specimen as Hipparion sp. It was collected in 2016 by Julie Niederkorn from MMBQ spoil piles. The stage of evolution of this hypsodont tooth is far too advanced to be from the Maysville L.F. Compared to other late Miocene–Pliocene hipparionine (three-toed) horses, USNM 639777, collected by Julie Niederkorn, has an oval, well-isolated protocone. The tooth is larger than those of small species such as Nannippus aztecus Mooser, Reference Mooser1968. Likewise, it is smaller than either Cormohipparion emsliei Hulbert, Reference Hulbert1988, or Neohipparion eurystyle (Cope, Reference Cope1893), the latter of which also has a morphologically more advanced protocone. USNM 639777 compares favorably with teeth of Nannippus peninsulatus from the Santa Fe (e.g., UF 1143) and Ichetucknee rivers in northern Florida (MacFadden and Waldrop, Reference MacFadden and Waldrop1980, as N. phlegon [Hay, Reference Hay1899]). Nannippus peninsulatus is also recorded from Pamlico and Craven counties, North Carolina (Bruff et al., Reference Bruff, Bain, Chandler and Chandler2017, p. 190). Nannippus peninsulatus is primarily known throughout much of North America during the Blancan NALMA (https://paleobiodb.org). This species therefore seems to be the best allocation for USNM 639777.
Bear (Ursidae), USNM 639769
This medial phalanx (Fig. 16.2, 16.3), collected by Julie Niederkorn, is not assigned to family, genus, or species (in https://collections.nmnh.si.edu/search/paleo/?irn=3447591). It was collected from spoil piles prior to when we worked the in-situ sediments in 2018 from the Haywood Landing Member, so its precise stratigraphic position is unknown. Bruff et al. (Reference Bruff, Bain, Chandler and Chandler2017, p. 168) identified this specimen as “Camelidae sp.” However, based upon comparisons with relevant specimens (in https://specifyportal.floridamuseum.ufl.edu/vp/), the morphology of this bone, and in particular, the triangular flange on the proximal articular surface of the bone, overall proportions, as well as the general morphology of the distal trochlear region, are not representative of camelids. Instead, this medial phalanx compares favorably with bears (Ursidae) (in https://www.floridamuseum.ufl.edu/vertpaleo/) from late Pleistocene localities such as Devil’s Den, Florida (e.g., UF 8778). USNM 639769 is a white plaster cast, and, as such, little can be said about its mode of fossilization. It is conceivable that this specimen could represent an earlier ursid, whose fossil record in North America extends back to the Early Miocene (Hunt, 1998; Qiu, Reference Qiu2003; Tedford et al., Reference Tedford, Albright, Barnosky, Ferrusquia-Villafranca, Hunt, Storer, Swisher, Voorhies, Webb, Whistler and Woodburne2004), but with so little to evaluate its age and comparative specimens in UF Vertebrate Paleontology collection, USNM 639769 is placed on record here within this family based on comparison with specimens from Devil’s Den, Florida.
Odocoileus virginianus (Cervidae), USNM 639775
This medial phalanx (Fig. 16.4, 16.5), collected by Shelda Aultman, is also not identified to family, genus, or species (in https://collections.nmnh.si.edu/search/paleo/?irn=3447591). Bruff et al. (Reference Bruff, Bain, Chandler and Chandler2017, p. 208) illustrated this bone as “Artiodactyla? sp.” The overall proportions and general morphology of the proximal articular surface and distal trochlear region or the phalanx are not representative of camelids. Our comparisons indicate that USNM 639775 is nearly identical to the same bones of fossil Odocoileus virginianus (white-tailed deer) from the northern Florida rivers (e.g., UF 10658) of Late Pliocene and Pleistocene (Blancan through Rancholabrean) age. It also is nearly identical to the same element of extant skeletons of O. virginianus in the UF Mammalogy Collection (UF 25430 from Collier County, Florida, although USNM 639775 could conceivably be from a Holocene individual, because its state of preservation suggests that it is a fossil).
Age, provenience, and final comment
Given the known biochronological ranges of these specimens, N. peninsulatus (USNM 63977) is most likely from either the Duplin or Croatan formations. On the other hand, the bear (USNM 639769) and deer (USNM 639775) potentially have longer temporal ranges. They could be from the Neogene formations, although their provenience from the unstudied later Pleistocene deposits (Fig. 1) also cannot be ruled out. In summary, these three specimens are not part of the Maysville L.F. at MMBQ. As collecting continues at MMBQ, particularly from float and spoil piles, age determinations for land mammals, particularly those that are fragmentary and with long temporal ranges, will continue to be a challenge. Geochemical techniques, such as REE (rare earth elements) discrimination may help to sort temporally mixed fossil assemblages, as well as ground truth our interpretations posited here.





















