Introduction
Hermit crabs (superfamily Paguroidea Latreille, Reference Latreille1802) are an interesting and diverse group of decapod crustaceans with a widely distributed but fragmentary fossil record (i.e., Via, Reference Via1959; Beschin et al., Reference Beschin, Busulini, De Angeli and Tessier2002, Reference Beschin, De Angeli, Checchi and Zarantonello2005, Reference Beschin, Busulini and Tessier2010, Reference Beschin, De Angeli, Checchi and Zarantonello2012; De Angeli et al., Reference De Angeli and Caporiondo2009; Garassino et al., Reference Garassino, De Angeli and Pasini2009a, Reference Garassino, De Angeli and Pasinib; Pasini and Garassino, Reference Pasini and Garassino2010a, Reference Pasini and Garassinob, Reference Pasini and Garassino2011; Fraaije et al., Reference Fraaije, van Bakel, Iserbyt and Jagt2011, Reference Fraaije, van Bakel and Jagt2015, Reference Fraaije, Beschin, Busulini, Tessier, Jagt, van Bakel, Jagt, Fraaije, van Bakel, Donovan and Mellish2020; Schweigert et al., Reference Schweigert, Fraaije, Havlik and Nützel2013; Fraaije, Reference Fraaije2014; Garassino et al., Reference Garassino, De Angeli, Pasini and Hyžný2014; Hyžný et al., Reference Hyžný, Fraaije, Martin, Perrier and Sarr2016; De Angeli and Caporiondo, Reference De Angeli and Caporiondo2017; Ferratges et al., Reference Ferratges, Zamora and Aurell2020, Reference Ferratges, Artal and Zamora2021a; Mironenko, Reference Mironenko2020; Ossó, Reference Ossó2020; Pasini et al., Reference Pasini, Garassino, Nyborg, Dunbar and Fraaije2020) that extends back to the Jurassic (see Fraaije et al., Reference Fraaije, van Bakel, Jagt, Charbonnier, Schweigert, Garcia and Valentin2022). This is due in part to their highly specialized morphology with poorly mineralized pleon adapted to life inside empty shells or other cavities (e.g., Lemaitre, Reference Lemaitre1989, Reference Lemaitre1990; Walker, Reference Walker1992; de Forges et al., Reference de Forges, Chan, Corbari, Lemaitre, Macpherson, Ahyong and Ng2001). After death, disarticulation occurs rapidly, and the fossil record of this group is represented mostly by isolated propodi and chelae, which are the harder and more resistant parts (see Klompmaker et al., Reference Klompmaker, Portell and Frick2017).
Eocene outcrops in Europe have provided a rich diversity of hermit crabs, especially in the middle and late Eocene, concentrated in reef environments from Italy (Beschin et al., Reference Beschin, Busulini, De Angeli and Tessier2007, Reference Beschin, Busulini and Tessier2015, Reference Beschin, Busulini, Fornaciari, Papazzoni and Tessier2018, Reference Beschin, Busulini, Tessier and Zorzin2019; Tessier et al., Reference Tessier, Beschin and Busulini2011) and Hungary (Müller and Collins, Reference Müller and Collins1991) and siliciclastic prodelta environments from Italy (De Angeli and Caporiondo, Reference De Angeli and Caporiondo2017). By contrast, early Eocene material is rarer and concentrated in only a few localities (see Fraaije et al., Reference Fraaije, van Bakel, Iserbyt and Jagt2011; Beschin et al., Reference Beschin, Busulini, Tessier and Zorzin2016; Fraaije and Polkowsky, Reference Fraaije and Polkowsky2016; Ferratges et al., Reference Ferratges, Zamora and Aurell2021b). However, Paleocene records of paguroids are scarce, and hermit crab assemblages of this age remain largely understudied (see Jakobsen et al., Reference Jakobsen, Fraaije, Jagt and van Bakel2020 and references therein).
During the Paleocene–Eocene, the southern Pyrenean basin corresponded to an elongated gulf located in tropical latitudes (Hay et al., Reference Hay, Barrera and Johnson1999), resulting in a biodiversity hotspot of several marine invertebrates, including decapod crustaceans, and the development of coral-reef environments (Ferratges et al., Reference Ferratges, Zamora and Aurell2021b). In this sense, the early Eocene seems to be an important period of diversification of hermit crabs, with the appearance of several modern families. Here we describe eight taxa of paguroids from the middle Ypresian (lower Eocene) associated with reef environments from the Ramals outcrop in the Pyrenees of Huesca, Spain. This locality has provided a great diversity of other decapod crustaceans (Artal and Via, Reference Artal and Via1989; Artal and Castillo, Reference Artal and Castillo2005; Artal and van Bakel, Reference Artal and van Bakel2018a, Reference Artal and van Bakelb; Ferratges et al., Reference Ferratges, Zamora and Aurell2019, Reference Ferratges, Zamora and Aurell2021b; Artal et al., Reference Artal, Ferratges, van Bakel and Zamora2022), but paguroids remained undescribed until the present study.
The aim of the present study includes the description of new paguroids discovered in the Serraduy Formation (Ypresian, lower Eocene) from the southern Pyrenees (Spain). This important association shows diverse paguroids associated with a reef environment. The presence of complete chelae allows comparison with both modern and fossil representatives of the group and enlarges the general knowledge of the European fossil record of Paguridae.
Locality, materials, and methods
Locality
The material described herein was collected from the lower Eocene (middle Ypresian) Serraduy Formation of the Tremp-Graus Basin. All specimens were collected from the same levels described by Ferratges et al. (Reference Ferratges, Zamora and Aurell2021b) and Artal et al. (Reference Artal, Ferratges, van Bakel and Zamora2022).
Materials
The studied material comprises 130 specimens represented by isolated left and right propodi belonging to eight genera and eight species, from which six are formally named. The material included in the present study was collected from the outcrop that exposes the transition between the reef limestones and the overlying Riguala Marls at a locality known as “Barranco de Ramals” (see Ferratges et al., Reference Ferratges, Zamora and Aurell2021b for further information).
Some of this material (50 isolated propodi, 5.49% of the total decapod crustacean assemblage) was recovered during a paleoecological study of the area (see Ferratges et al., Reference Ferratges, Zamora and Aurell2021b). The remaining specimens (80 isolated propodi and chelae) were studied in historical museum collections (MGSB). The studied chelae are well preserved, usually with their cuticle and without deformation.
Left and right chelae showing apparent homochely, as in the new species included in Clibanarius, Parapetrochirus, and Dardanus, have been considered to belong to the same taxon. In the case of taxa with probably asymmetric chelae (heterochely), assignment to the same taxon has been discarded due to very different ornamentations between different genera and to the fact that none of the known representatives of these genera fit with the other chelae collected in the same area. This is the case of the genera Petrochirus Stimpson, Reference Stimpson1859, Eocalcinus Via, Reference Via1959, Pagurus Fabricius, Reference Fabricius1775, Paguristes Dana, Reference Dana1852, and Anisopagurus McLaughlin, Reference McLaughlin1981.
Methods
The specimens were prepared using a Micro Jack 2 air scribe (Paleotools) and binocular magnifying. They were later photographed dry and coated with ammonium chloride sublimate. Detailed photography of the cheliped surfaces was made using a Nikon d7100 camera (Nikon, Tokyo, Japan) with a 60 mm macro lens.
Repositories and institutional abbreviations
The specimens are deposited in the Museo Geológico del Seminario de Barcelona (MGSB) and the Museo de Ciencias Naturales de la Universidad de Zaragoza (Spain) (MPZ). The material deposited in MPZ was collected under permit EXP: 032/2018 from the Servicio de Prevención, Protección e Investigación del Patrimonio Cultural (Gobierno de Aragón). The material deposited in MGSB was collected in the early 1980s and is housed within the historical collection of the Seminario Conciliar de Barcelona.
Systematic paleontology
Systematic classification follows McLaughlin (Reference McLaughlin2003), McLaughlin et al. (Reference McLaughlin, Lemaitre and Sorhannus2007; Reference McLaughlin, Komai, Lemaitre and Rahayu2010), and Fraaije et al. (Reference Fraaije, van Bakel, Jagt, Charbonnier, Schweigert, Garcia and Valentin2022). For the morphological terminology of chelipeds, see Figure 1.

Figure 1. Simplified anatomical scheme of cheliped morphotypes of paguroids. (1, 2) Frontal view of two different morphotypes. (3) Lateral view. CPA = carpo-propodus articulation.
Order Decapoda Latreille, Reference Latreille1802
Infraorder Anomura MacLeay, Reference MacLeay and Smith1838
Superfamily Paguroidea Latreille, Reference Latreille1802
Family Diogenidae Ortmann, Reference Ortmann1892
Genus Clibanarius Dana, Reference Dana1852
Type species
Cancer clibanarius Herbst, 1791 (Herbst, Reference Herbst1791–1796).
Fossil species included
C. sossanensis (De Angeli and Caporiondo, Reference De Angeli and Caporiondo2009); C. cecconi (De Angeli and Caporiondo, Reference De Angeli and Caporiondo2017); C. isabenaensis n. sp.
Clibanarius isabenaensis new species
Figure 2
Type material
The holotype is MGSB77625, a near-complete, well-preserved left chela retaining cuticle. There are three paratypes (MGSB85955, MPZ 2021/30, MPZ 2022/1), which lack the dactylus.
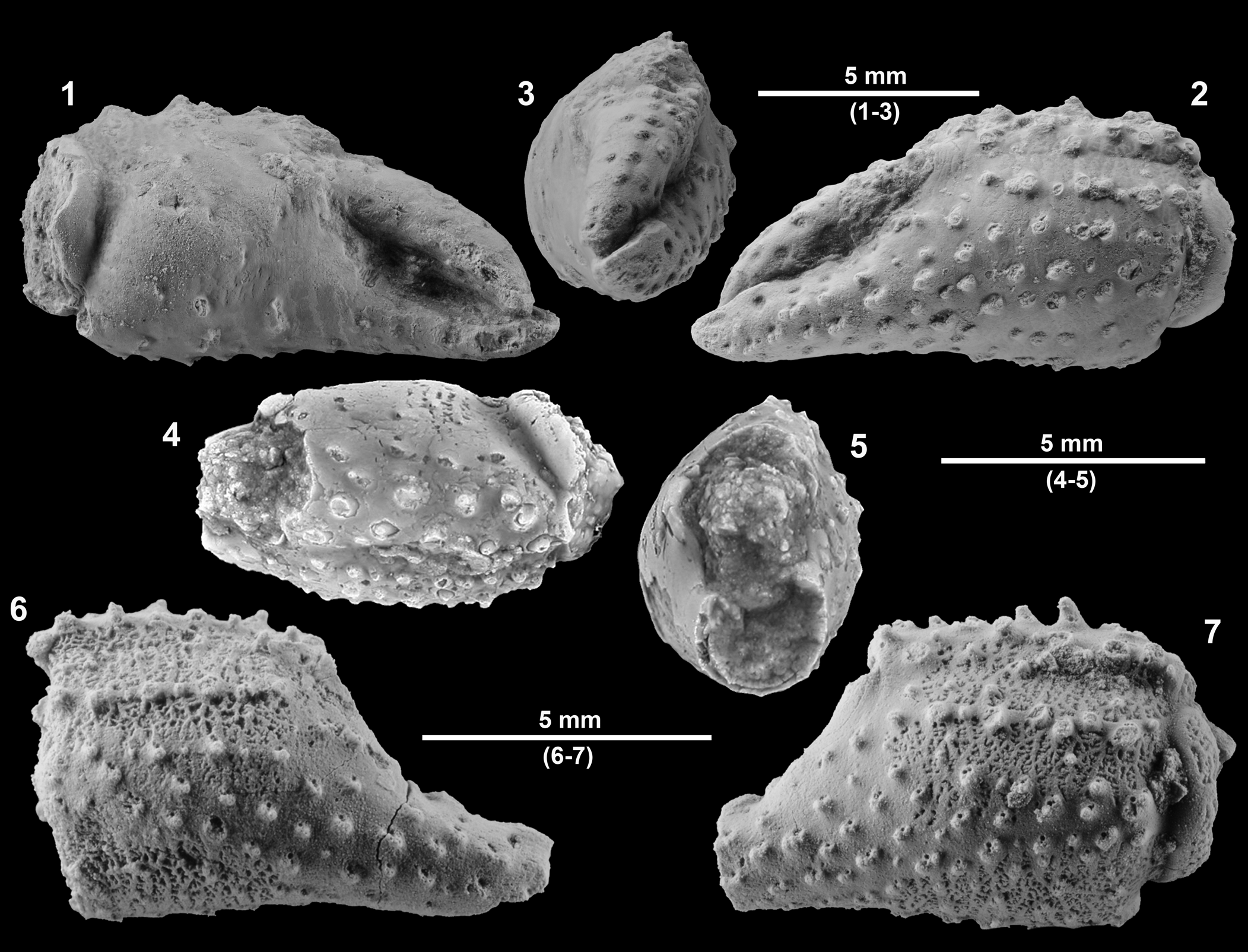
Figure 2. Clibanarius isabenaensis n. sp. (1–3) Holotype MGSB77625: (1) lateral view of inner side of left chela; (2) frontal view; (3) outer side lateral view of left chela. (4, 5) Paratype (MGSB85955), left chela: (4) upper view (5) frontal view. (6) Paratype MPZ 2021/30, lateral view of outer side of right chela. (7) Paratype MPZ 2022/1, lateral view of outer side of left chela.
Diagnosis
Small left and right chela. Right and left propodus with slightly tilted carpo-propodus articulation, oriented at angle over 50°. Palm anteriorly convergent. Both propodi of similar size and shape (homochely). Two rows of conical spines on upper margin. Four rows of spiny granules on outer surface of palm. Inner surface of palm smooth. Fingers slender, rounded, elongated, bearing granules and setal pits of large size. Occlusal margin with acute outer sides.
Description
Small left and right chelae of presumably similar shape and size. Palm subrectangular, somewhat longer than high. Complete propodus about 13.0 mm long, 7.0 mm palm length, and 6 mm palm height. Oval cross section. Inner surface fairly convex, nearly smooth. Outer surface densely granular, bearing four principal rows of spinose tubercles with setal pits near the base, directed upward. Upper margin with two rows of conical spines. Lower margin straight, rounded, with small conical granules directed forward. Posterior part of palm with prominent groove on both inner and outer surfaces, probably related to the articulation with the carpus. Fingers long, circular in cross section, slender, curved, with broad space between their occlusal margins. Large setal pits on fixed finger, of similar size and randomly distributed (Fig. 2).
Etymology
The specific name comes from Isábena, the municipality of the province of Huesca where the material was collected.
Other material examined
Ten additional specimens MGSB85956a–j and five additional specimens at MPZ 2022/2–6. All the examined materials have a similar size.
Remarks
The new species shows characteristics of the palm typical of the genus Clibanarius, as well as the presumed homochely, the small and similar size of both chelae, and the outer surface covered by small spines. Nevertheless, some taxonomic doubts exist with respect to species of the genus Clibanarius that are closely related to each other, and in some cases, this strong morphological similarity has raised questions about their status as separate species (McLaughlin et al., Reference McLaughlin, Komai, Lemaitre and Rahayu2010). In most extant species, the fingers present a robust, stout shape, being strongly thick and clearly short (Sánchez and Campos, Reference Sánchez and Campos1978; McLaughlin, Reference McLaughlin2003; McLaughlin et al., Reference McLaughlin, Lemaitre and Sorhannus2007, Reference McLaughlin, Komai, Lemaitre and Rahayu2010; Negri et al., Reference Negri, Lemaitre and Mantelatto2014). In almost all modern species included in the genus Clibanarius, the occlusal margins of the fingers are straight, with no gap between them (with some exceptions such as C. antillensis Stimpson, Reference Stimpson1859 and C. ambonensis Rahayu and Forest, Reference Rahayu and Forest1992). Clibanarius isabenaensis n. sp. exhibits longer and thinner fingers than the most modern representatives of the genus Clibanarius, with a curved dactylus and pollex, occlusal margin curved, with a wide gap between the fingers. However, we consider that the similarities presented by the new species justify inclusion in this genus.
Some species included in the genus Paguristes are similar to the new species, including several modern and fossil species (i.e., Müller and Collins, Reference Müller and Collins1991; Blow and Manning, Reference Blow and Manning1996; Beschin et al., Reference Beschin, De Angeli, Checchi and Zarantonello2005, Reference Beschin, Busulini, De Angeli and Tessier2007, Reference Beschin, Busulini, Fornaciari, Papazzoni and Tessier2018; De Angeli and Caporiondo, Reference De Angeli and Caporiondo2009, Reference De Angeli and Caporiondo2017; Garassino et al., Reference Garassino, De Angeli and Pasini2009b). However, modern representatives of the genus Paguristes originally included a large number of morphologically different taxa, and currently, the genus has been split into several less-variable genera (see McLaughlin et al., Reference McLaughlin, Komai, Lemaitre and Rahayu2010). Unfortunately, most diagnostic criteria used for modern species are not preserved in the fossil record. In any case, extant species assigned to Paguristes show certain differences from the new species: (1) heterochely; (2) shorter and more robust fingers; (3) setal pits tend to show a different distribution from that of the material assigned to Clibanarius isabenaensis n. sp. (grouping of several setal pits in front of the tubercles, oriented distally, instead of a large setal pit oriented obliquely upward). Furthermore, the extant species of Paguristes do not present tubercle alignment as in the new species (i.e., Rahayu and McLaughlin, Reference Rahayu and McLaughlin2006; Rahayu, Reference Rahayu2007; Komai, Reference Komai2010; McLaughlin et al., Reference McLaughlin, Komai, Lemaitre and Rahayu2010). The fossil species Paguristes cecconi De Angeli and Caporiondo, Reference De Angeli and Caporiondo2017 shows a clear affinity with the material studied here. Nevertheless, P. cecconi differs from C. isabenaensis n. sp. by having a less elongate shape and fewer and more robust spiny tubercles on the upper margin (see De Angeli and Caporiondo, Reference De Angeli and Caporiondo2017, p. 15–16, fig. 7, t. 3). Furthermore, C. isabenaensis n. sp. has slightly less convergent upper and lower margins than P. cecconi. The species Paguristes sossanensis De Angeli and Caporiondo, Reference De Angeli, Garassino and Pasini2009 also shows similarities with C. isabenaensis n. sp. in the general shape of the chela and distribution of the tubercles (see De Angeli and Caporiondo, Reference De Angeli, Garassino and Pasini2009, p. 24–25, figs. 2, 3). However, P. sossanensis shows a more globose morphology, smaller tubercles on the outer surface, reduced spines on the upper margin, and a shorter and more robust fixed finger. For these reasons, we consider that the species P. cecconi and P. sossanensis should be assigned to the genus Clibanarius.
Genus Petrochirus Stimpson, Reference Stimpson1859
Type species
Pagurus granulatus Olivier, Reference Olivier1811 (= Cancer bahamensis Herbst, 1796 (for 1791 in Herbst, Reference Herbst1782–1804]), by original designation.
Fossil species included
Petrochirus bahamensis (Herbst, 1791); P. bouvieri Rathbun, Reference Rathbun1919a; P. diogenes (Linnaeus, Reference Linnaeus1758); P. inequalis Rathbun, Reference Rathbun1919b; P. mezi (Lőrenthey, Reference Lőrenthey1909); P. minutus Beschin et al., Reference Beschin, Busulini, Tessier and Zorzin2016; P. poscolensis Beschin et al., Reference Beschin, De Angeli, Checchi and Mietto2006; P. priscus (Brocchi, Reference Brocchi1883); P. sanctilazzari Baldanza et al., Reference Baldanza, Bizzarri, Famiani, Pasini, Garassino and De Angeli2014; P. savii Beschin et al., Reference Beschin, De Angeli, Checchi and Zarantonello2012; P. taylori Rathbun, Reference Rathbun1935.
?Petrochirus sp.
Figure 3
Description
Propodus length: 22.0 mm; palm length: 14.7 mm; palm height: 14.0 mm. Palm subrectangular, outer surface of palm densely coarsely granulate; inner surface less ornamented with granules. On the outer surface, more dense and coarse tubercles; on the inner surface, more numerous in the upper portion; lower portion nearly smooth. Both surfaces convex. Palm sigmoidal in cross section. Upper margin with four prominent spines surrounded by other smaller spines, irregularly distributed. Upper and lower margins of propodus straight (Fig. 3). Incomplete remains of fixed finger exhibit a robust construction and strong occlusal molariform teeth.

Figure 3. ?Petrochirus sp. (1–3) Right cheliped (specimen MPZ 2022/10): (1) lateral view of outer side; (2) frontal view; (3) upper view.
Material examined
Four specimens corresponding to one isolated propodus (MPZ 2022/10) and three movable fingers (MPZ 2022/11–12, MPZ 2022/59).
Remarks
This taxon is characterized by a subquadrate palm, with fairly convex inner and outer surfaces and densely covered by unevenly spaced granules. The fixed finger is not complete, but the first portion suggests it is robust, rounded, and elongated. Numerous incomplete or badly preserved chelae (Portell and Agnew, Reference Portell and Agnew2004; Vega et al., Reference Vega, Nyborg, Coutiño and Hernández-Monzón2008; Collins et al., Reference Collins, Donovan and Stemann2009a, Reference Collins, Portell and Donovanb; Bermudez et al., Reference Bermúdez, Vega-Sandoval and Vega2017; Luque et al., Reference Luque, Nyborg, Alvarado-Ortega and Vega2020) have been traditionally assigned to Petrochirus mainly on the basis of a subrectangular shape and the squamous or pavement-like ornamentation (see Beschin et al., Reference Beschin, Busulini, De Angeli and Tessier2002; Todd and Collins, Reference Todd and Collins2005; Vega et al., Reference Vega, Nyborg, Coutiño, Solé and Hernández-Monzón2009; De Angeli and Caporiondo, Reference Bermúdez, Vega-Sandoval and Vega2017; Luque et al., Reference Luque, Schweitzer, Santana, Portell, Vega and Klompmaker2017). The most similar fossil remains are from P. savii from Italy. Major differences are the coarser, larger granules on the outer surface and the bigger, more numerous granules on the inner surface of the palm in the Spanish form. The Italian form is characterized by a more elongate, subrectangular palm; an outer surface with smaller granules; an inner surface of the palm smooth, reticulate, with very few granules (De Angeli and Caporiondo, Reference Bermúdez, Vega-Sandoval and Vega2017).
Genus Parapetrochirus Ferratges, Artal, and Zamora, Reference Ferratges, Artal and Zamora2021a
Type species
Parapetrochirus robustus Ferratges, Artal, and Zamora, 2021 (Ferratges et al., Reference Ferratges, Artal and Zamora2021a).
Fossil species included
P. robustus Ferratges, Artal, and Zamora, 2021; P. serratus n. sp.
Parapetrochirus serratus new species
Figure 4
Type material
The holotype is MPZ 2022/7, a well-preserved left propodus, with cuticle preserved; there are also three paratypes (two right propodi and one isolated dactylus): MGSB77621a–c.

Figure 4. Parapetrochirus serratus n. sp. (1–4) Holotype MPZ 2022/7, left chela: (1) lateral view of outer side; (2) frontal view; (3) upper view; (4) lower view. (5) Paratype MGSB77621a, lateral view of outer side of right cheliped. (6–9) Paratype MGSB77621b, right chela: (6) lateral view of outer side; (7) upper view; (8) frontal view; (9) inner side lateral view. (10) Occlusal margin of isolated dactylus (paratype MGSB77621c). (11) Detail of the capsulated setal pits of the occlusal margin of dactylus.
Diagnosis
Upper and lower margins of the palm notably ridged; oblique strong ridge on the medial portion of the inner surface of the palm; occlusal margin of the fixed finger bearing three molariform teeth, various small setal pits, and two relatively large elliptical depressions with numerous setal pits. The propodi are of similar size and shape (homochely).
Description
Propodus length: 19.5 mm; palm length: 12.8 mm; palm height: 13.9 mm of holotype. Upper and lower margins of the palm strongly ridged, angular, developed as a strong oblique ridge in the inner margin. Upper margin straight, becoming higher proximally; lower margin straight, also higher proximally. Both margins with dentiform tubercles. Inner and outer surface of palm densely tuberculated, covered with closely spaced squamose granules. Palm with convex upper and lower margins, triangular in cross section, longer than high, with the upper and lower margins straight, subparallel, somewhat inclined, outer portion only somewhat convex, nearly flat; both margins angular, keeled, with notable conical denticles directed forward. Propodi are of similar size and shape (homochely), forming a circular shield when joined (Fig. 4.1, 4.5). The ornamentation of the inner and outer surfaces consists of squamose closely spaced tubercles. Fixed finger robust, triangular in cross section, straight. Dactylus robust; the occlusal edge is concave, smooth, bearing up to three molariform teeth, about four small setal pits, and two large depressions near the tip that exhibit multiple, numerous small setal pits (Fig. 4.10, 4.11).
Etymology
From the Latin serratus, referring to its serrated margins.
Other material examined
Eleven additional specimens at MGSB85957a–k, and three specimens at MPZ (one left dactylus: MPZ 2021/37; two fragments: MPZ 2022/8–9. All the examined material has similar size to the type material.
Remarks
Incomplete remains of fossil paguroids with squamous ornamentation have usually been assigned to the genus Petrochirus (i.e., Portell and Agnew, Reference Portell and Agnew2004; Todd and Collins, Reference Todd and Collins2005; Vega et al., Reference Vega, Nyborg, Coutiño and Hernández-Monzón2008; Bermúdez et al., Reference Bermúdez, Vega-Sandoval and Vega2017; Luque et al., Reference Luque, Schweitzer, Santana, Portell, Vega and Klompmaker2017, Reference Luque, Nyborg, Alvarado-Ortega and Vega2020). Some are recorded as extant species (i.e., Todd and Collins, Reference Todd and Collins2005; Collins et al., Reference Collins, Donovan and Stemann2009a, Reference Collins, Portell and Donovanb; Luque et al., Reference Luque, Schweitzer, Santana, Portell, Vega and Klompmaker2017). Petrochirus Stimpson, Reference Stimpson1859 is characterized by globular chelipeds with elongate and subrectangular palms covered by numerous granules on the inner and outer surfaces. However, the genus Parapetrochirus is characterized by angular, strongly ridged upper and lower margins of the palm and a strong oblique ridge situated in the medial portion of the inner surface and margins bearing strong conical teeth. Some rather complete chelae from Italy identified as Petrochirus savii (De Angeli and Caporiondo, Reference De Angeli and Caporiondo2017) and Petrochirus sanctilazzari Baldanza et al., Reference Baldanza, Bizzarri, Famiani, Pasini, Garassino and De Angeli2014 appear morphologically similar to the Spanish genus Parapetrochirus. Petrochirus savii presents striking similarities, mainly in the occlusal margins. The lower occlusal margin of Petrochirus savii presents some characters that are nearly identical to Parapetrochirus serratus n. sp., such as molariform teeth and deep elliptical depressions (De Angeli and Caporiondo, Reference De Angeli and Caporiondo2017). Differences in Petrochirus savii from Parapetrochirus serratus are palm more elongate; subrectangular; upper and lower margin of palm rounded, not crested; different ornamentation; near absence of granules on the inner portion; smaller granules, not pavement-like, on the outer portion. Main differences in Petrochirus sanctilazzari from Parapetrochirus serratus are a more elongated palm and more rounded lower and upper margins in the former.
The Mexican Petrochirus sp. from the lower Eocene (Vega et al., Reference Vega, Nyborg, Coutiño and Hernández-Monzón2008) shows some similarities to Parapetrochirus serratus n. sp. such as upper and lower margins bearing conical teeth, dense ornamentation, and a subrectangular propodus. The main differences in the Mexican specimen are: (1) absence of a strong oblique ridge on the inner surface; (2) lower margin strongly arched; (3) outer surface of the palm scarcely granulated (see Vega et al., Reference Vega, Nyborg, Coutiño and Hernández-Monzón2008).
Parapetrochirus serratus n. sp. shows similarities to Calcinus agonensis Beschin et al., Reference Beschin, De Angeli, Checchi and Zarantonello2005 in the general outline and ornamentation of the chela. However, the new species presents more weakly developed tubercles on the upper margin and a more serrated lower margin. In addition, modern representatives of the genus Calcinus show a great diversity of shapes and need deep systematic review. In any case, the modern forms assigned to the genus Calcinus present clear differences from P. serratus n. sp. such as: (1) evident heterochely and (2) globose chelae, not rounded or opercular as is the case with fossil material (i.e., Forest, Reference Forest1958, p. 4–7, 9–12, figs. 6–12; Haig and McLaughlin, Reference Haig and McLaughlin1984, p. 109–110, 112, 117, figs. 1, 2; Poupin, Reference Poupin1997, figs. 4–7; Asakura and Tachikawa, Reference Asakura and Tachikawa2000, p. 270, 275, figs. 2, 6; Asakura, Reference Asakura2002, p. 29, 32, 34–35, 37, 41, 47, 51–52, 56–57, 59, 64–65, figs. 2–6, 8, 10, 13–16, 18–21; Poupin and Lemaitre, Reference Poupin and Lemaitre2003, p. 5, 7, figs. 1–3, 5).
The species Parapetrochirus robustus from the upper Ypresian of Huesca (Spain) also shows similarities with the new species in the ornamentation; inner and outer surface of the palm densely tuberculated, covered with squamose granules; robust fixed finger; lower margin arched proximally, and keeled in the distal portion (see Ferratges et al., Reference Ferratges, Artal and Zamora2021a). However, P. serratus n. sp. has a much more compact shape, with an oval outline, less compressed in the lower zone, convergent upper and lower margins, not divergent as in P. robustus, and less dense ornamentation. In addition, the new species presents both chelipeds with very similar morphology.
Family Annuntidiogenidae Fraaije, Reference Fraaije, Krzemiński, van Bakel, Krzemińska and Jagt2014
Genus Paguristes Dana, Reference Dana1852
Type species
Paguristes hirtus Dana, Reference Dana1852 by subsequent designation of Stimpson, Reference Stimpson1859.
Fossil species included
Paguristes baldoensis Garassino, De Angeli, and Pasini, Reference De Angeli and Caporiondo2009 (Garassino et al., Reference Garassino, De Angeli and Pasini2009b); P. cecconi De Angeli and Caporiondo, Reference De Angeli and Caporiondo2017; P. chipolensis Rathbun, Reference Rathbun1935; P. clampensis De Angeli and Caporiondo, Reference De Angeli and Caporiondo2017; P. cserhatensis Müller, Reference Müller1984; P. florae Collins, Fraaye, and Jagt, Reference Collins, Fraaye and Jagt1995; P. hokoensis Schweitzer and Feldmann, Reference Schweitzer and Feldmann2001; P. johnsoni Rathbun, Reference Rathbun1935; P. lineatuberculatus Beschin et al., Reference Beschin, De Angeli, Checchi and Mietto2006; P. liwinskii Fraaije, Van Bakel, and Jagt, Reference Fraaije, van Bakel and Jagt2015; P. mexicanus (Vega et al., Reference Vega, Cosma, Coutiño, Feldmann, Nyborg, Schweitzer and Waugh2001); P. michikoae Karasawa and Fudouji, Reference Karasawa and Fudouji2018; P. oligotuberculatus Müller and Collins, Reference Müller and Collins1991; P. ouachitensis Rathbun, Reference Rathbun1935; P. paucituberculatus Beschin, Busulini, and Tessier in Beschin et al., Reference Beschin, Busulini, Tessier and Zorzin2016; P. prealpinus Beschin et al., Reference Beschin, De Angeli, Checchi and Zarantonello2005; P. santamartaensis Feldmann, Tshudy, and Thomson, Reference Feldmann, Tshudy and Thomson1993; P. sossanensis De Angeli and Caporiondo, Reference De Angeli, Garassino and Pasini2009; P. subaequalis (Rathbun, Reference Rathbun1926); P. teruakii Karasawa and Fudouji, Reference Karasawa and Fudouji2018; P. wheeleri Blow and Manning, Reference Blow and Manning1996; P. whitteni Bishop, Reference Bishop1983 (modified from Schweitzer et al., Reference Schweitzer, Feldmann, Garassino, Karasawa and Schweigert2010 and Fraaije et al., Reference Fraaije, van Bakel and Jagt2015).
Remarks
The genus Paguristes Dana, Reference Dana1852 was previously considered in the family Diogenidae (sensu lato), a position that was revised by Fraaije (Reference Fraaije2014) and Fraaije et al. (Reference Fraaije, van Bakel and Jagt2017). These authors proposed its inclusion in a new family (Annuntidiogenidae Fraaije, Reference Fraaije2014). Paguroid phylogeny is not in the scope of this paper, and we follow at this moment placement of the genus Paguristes in the Annuntidiogenidae as proposed by Fraaije (Reference Fraaije2014) and Fraaije et al (Reference Fraaije, van Bakel and Jagt2017, Reference Fraaije, van Bakel, Jagt, Charbonnier, Schweigert, Garcia and Valentin2022).
Paguristes perlatus new species
Figure 5
Type material
The holotype, MPZ 2022/38, is a left propodus (propodus length without fixed finger: 8.0 mm; palm length: 7.2 mm; palm height: 7.0 mm).

Figure 5. Paguristes perlatus n. sp. (1–4) Holotype (MPZ 2022/38), left chela: (1) lateral view of outer side; (2) frontal view; (3) upper view. (4) Detail of the distribution of the setal pits. Specimens whitened with ammonium chloride sublimate.
Diagnosis
Palm subquadrate; upper margin short, with strong conical teeth; lower margin fairly concave. Outer surface convex, densely granular. Inner surface with strong ridge. Upper portion of the inner surface concave; lower portion concave. Carpo-propodus articulation oblique. Fingers curving inward laterally when seen from dorsal view.
Description
Chela of small size, palm subquadrate, slightly higher than long; outer surface concave, densely covered by evenly spaced perliform granules; inner surface bearing a strong medial ridge (inner margin), the lower portion densely granular. Inner margin rounded, strongly concave. Propodus with concavity on the upper portion of inner surface when seen from frontal view, fingers curving laterally. Lower portion of the inner surface concave. Upper margin short, bearing strong conical teeth. Lower margin longer, notably concave. Carpo-propodus articulation fairly oblique, short (Fig. 5).
Etymology
The name refers to the characteristic perlated tubercles on the outer surface of the palm.
Remarks
The genus Paguristes is morphologically diverse in modern ecosystems (Rahayu, Reference Rahayu2006). Members of Paguristes in the fossil record are diagnosed by various characteristics (i.e., Beschin et al., Reference Beschin, De Angeli, Checchi and Zarantonello2012, Reference Beschin, Busulini, Tessier and Zorzin2016): carpus short, highest distally, with concave, arcuate lower margin and ornamented with spines and nodes; palm short, shortest along the upper margin, ornamented with numerous tubercles and spines; fixed finger stout and very high proximally. Because the features of the chelipeds of the fossil material are similar to members of Paguristes, the new material is placed tentatively within this genus.
Paguristes perlatus n. sp. exhibits a short upper margin of the palm bearing strongly marked conical teeth. The lower margin is fairly concave with a marked convexity in the proximal portion. Palm robust, globular, somewhat higher than long. Both surfaces are convex, the outer surface densely covered by pearled granules, the inner surface with an oblique ridge, the lower portion with numerous granules. Carpo-propodus articulation oblique. All the characters fit with the general morphological characteristics of the genus Paguristes. Major differences from the extant species are the ornamentation of the palm, which exhibits conical spines and the usually less concave lower margin of palm (Provenzano, Reference Provenzano1965; Campos and Sanchez, Reference Campos and Sánchez1995; Manjón et al., Reference Manjón, García and Martínez2002; Lima and Santana, Reference Lima and Santana2017). The morphologically closest fossil form is Paguristes prealpinus, which shares the main morphological characteristics described here. Major differences from the new species are the more subrectangular shape of the palm, the upper margin with less marked conical teeth; the concavity in the lower margin distally situated; granulation in the outer surface less marked and more irregularly distributed (Beschin et al., Reference Beschin, De Angeli, Checchi and Zarantonello2012; De Angeli and Caporiondo, Reference De Angeli and Caporiondo2017). Paguristes cecconi is assigned in this study to Clibanarius, as indicated in the preceding.
The fossil species Paguristes hokoensis, P. liwinskii, and P. teruakii exhibit the characteristic lateral curvature of the fingers from dorsal view, the conical teeth in the upper margin, and the granulated outer surface. However, P. hokoensis and P. teruakii have different ornamentation, a more elongated outline, strongly convergent upper and lower margins, and more rounded proximal lower margin (see Schweitzer and Feldmann, Reference Schweitzer and Feldmann2001, p. 193–195, fig. 13; Karasawa and Fudouji, Reference Karasawa and Fudouji2018, p. 26, fig. 2). Major differences in P. liwinskii are the coarse granulation of the palm, the oval outline with markedly convex lower margin, and the smoother inner surface (Fraaije et al., Reference Fraaije, van Bakel and Jagt2015, p. 590, fig. 1C).
Extant Paguristes consists of over 120 species (McLaughlin et al., Reference McLaughlin, Komai, Lemaitre and Rahayu2010; Komai et al., Reference Komai, Reshmi and Kumar2015). Several authors suggested extant Paguristes are mainly distributed in shallow-water areas of the temperate–tropical waters (i.e., Rahayu, Reference Rahayu2006; Rahayu and Forest, Reference Rahayu and Forest2009; Trivedi and Vachhrajani, Reference Trivedi and Vachhrajani2017).
Family Calcinidae Fraaije, Van Bakel, and Jagt, Reference Fraaije, van Bakel and Jagt2017
Genus Dardanus Paul'son, Reference Paul'son1875
Type species
Dardanus hellerii Paul'son, Reference Paul'son1875 by monotypy.
Fossil species included
D. arnoldi Rathbun, Reference Rathbun1926; D. arrosor (Herbst, 1796) (Herbst, Reference Herbst1782–1804); D. balaitus n. sp.; D. bayani Beschin et al., Reference Beschin, Busulini, Tessier and Zorzin2016; D. biordines Collins in Todd and Collins, Reference Todd and Collins2005; D. braggensis Beschin, Busulini, and Tessier, Reference Beschin, Busulini and Tessier2015; D. curtimanus Müller and Collins, Reference Müller and Collins1991; D. gemmatus (Milne Edwards, Reference Milne Edwards1848); D. hungaricus (Lörenthey in Lörenthey and Beurlen, Reference Lörenthey and Beurlen1929); D. impressus (De Haan, Reference De Haan and Siebold1833–1850); D. lauensis Rathbun, Reference Rathbun, Ladd and Hoffmeister1945; D. mediterraneus (Lörenthey, Reference Lőrenthey1909); D. mexicanus Vega et al., Reference Vega, Cosma, Coutiño, Feldmann, Nyborg, Schweitzer and Waugh2001; D. muelleri Karasawa and Inoue, Reference Karasawa and Inoue1992; D. squamatus Collins in Collins et al., 2009 (Collins et al., Reference Collins, Portell and Donovan2009b); D. substriatiformis (Lörenthey in Lörenthey and Beurlen, Reference Lörenthey and Beurlen1929).
Remarks
The genus Dardanus Paul'son, Reference Paul'son1875 was previously considered in the family Diogenidae (sensu lato), and its position was revised by Fraaije et al. (Reference Fraaije, van Bakel and Jagt2017). However, paguroid phylogeny is not in the scope of this paper; for consistency, we here follow Fraaije et al. (Reference Fraaije, van Bakel and Jagt2017, Reference Fraaije, van Bakel, Jagt, Charbonnier, Schweigert, Garcia and Valentin2022).
Dardanus balaitus new species
Figure 6
Type material
The holotype, MGSB77622, is a near-complete, well-preserved right propodus, retaining cuticle. There are two paratypes, one left propodus without dactylus, MGSB77623, and one isolated finger, MPZ 2021/36.

Figure 6. Dardanus balaitus n. sp. (1–3) Holotype MGSB77622, right chela: (1) lateral view of outer side; (2) frontal view; (3) upper view. (4, 5) Paratype MGSB77623, left chela: (4) lateral view of inner side; (5) lateral view of outer side. (6) Oblique interior view of the paratype MGSB77623. (7) Detail of the distribution of the setal pits. (8) Isolated dactylus (MPZ 2021/36).
Diagnosis
Elongated propodus; palm globular. Inner surface convex, lower portion with notable arched lobes, upper portion nearly smooth; lower portion with notable arched lobes bearing numerous setal pits. Outer surface convex, densely granulated, with spaced granules and tubercles, all of them bearing numerous setal pits on tips and in anterior portion.
Description
Propodus length: 20.0 mm; palm length: 12.0 mm; palm height: 13.0 mm of the holotype. Elongated propodus; palm globular, with rounded sides and margins. The inner surface bears an oblique inflation in the medial portion. Outer surface of the palm convex, ornamented with obliquely situated tubercles and oblique short or elongated raised lobes. The tubercles in the upper portion bearing one, two, three, or four setal pits, with several small setal pits in the anterior side. The lower portion of the outer surface with raised oblique lobes, the larger ones bearing about seven or eight setal pits on the tip and numerous, irregular, smaller setal pits on the anterior side. The setal pits on the anterior side are numerous and of irregular size; in the larger lobes, up to 18 smaller pits and up to eight larger pits. Inner surface smoother, less ornamented, but with large oblique rows of setal pits in the lower portion; the larger ones up to 20 irregular setal pits. Upper margin of palm with two rows of small conical teeth. Fixed finger with depressed, smooth occlusal margin; outer side of the finger with one strong, molariform tooth (Fig. 6).
Etymology
The specific name refers to the pre-Roman mythological character “Balaitús,” who lived in the Pyrenees and was dedicated to causing storms in the mountains.
Other material examined
Nineteen additional specimens (isolated propodi) in MGSB85958a–s and one isolated dactylus at MPZ 2021/36.
Remarks
The general morphology of chelae in the new taxon conforms with modern genus Dardanus Paul'son, Reference Paul'son1875 because the chelipeds are globular, with rounded margins and sides, with inner and outer surface fairly convex (i.e., Collins and Donovan, Reference Collins and Donovan2010; Garassino et al., Reference Garassino, De Angeli, Pasini and Hyžný2014) and because of the notable raised tubercles and lobes bearing numerous setal pits on the tips, and still more numerous setal pits in the anterior portion of each tubercle or elongated lobe. All of them appear obliquely situated, with the appearance of striations. This characteristic can be observed in both extant (Sánchez and Campos, Reference Sánchez and Campos1978; McLaughlin, Reference McLaughlin2003; McLaughlin et al., Reference McLaughlin, Lemaitre and Sorhannus2007, Reference McLaughlin, Komai, Lemaitre and Rahayu2010) and fossil taxa (Collins and Donovan, Reference Collins and Donovan2010; Fraaije et al., Reference Fraaije, van Bakel, Iserbyt and Jagt2011; Beschin et al., Reference Beschin, De Angeli, Checchi and Zarantonello2012; Garassino et al., Reference Garassino, De Angeli, Pasini and Hyžný2014). In addition, the fossil species assigned to Dardanus usually present long oblique or vertical ridges (i.e., Garassino et al., Reference Garassino, De Angeli, Pasini and Hyžný2014; Beschin et al., Reference Beschin, Busulini, Tessier, Fraaije and Jagt2021), with some exceptions such as D. colosseus Fraaije and Polkowsky, Reference Fraaije and Polkowsky2016 and D. vandeneeckhauti Fraaije et al., Reference Fraaije, van Bakel, Iserbyt and Jagt2011. Some of the distinctive characters of D. balaitus n. sp. are shared with the species D. arrosor, with robust chelae and oblique tuberculate ridges (McLaughlin et al., Reference McLaughlin, Lemaitre and Sorhannus2007, fig. 76). Nevertheless, the new species presents notable differences in the general shape of the chelipeds, being more rounded, and is distinct in having a peculiar distribution of tubercles and arched raised lobes, as it is the peculiar distribution of setal pits. The tubercles with one, two, three, or four setal pits on the tip; oblique arched lobes with up to eight setal pits on the tip: all tubercles and arched lobes with numerous and irregular setal pits in the anterior side (Fig. 6). The main difference with D. substriatus Garassino et al., Reference Garassino, De Angeli, Pasini and Hyžný2014 from the Pleistocene of Italy is the complete vertical striae on the outer surface of the propodus, which is absent in the new species (Garassino et al., Reference Garassino, De Angeli, Pasini and Hyžný2014).
Genus Eocalcinus Via, Reference Via1959
Type species
Eocalcinus eocenicus Via, Reference Via1959, by original designation.
Fossil species included
Eocalcinus albus Beschin, Busulini, and Tessier, Reference Beschin, Busulini and Tessier2010; E. cavus Beschin et al., Reference Beschin, Busulini, De Angeli and Tessier2002; E. eocenicus Via, Reference Via1959; E. gerardbretoni Ferratges, Artal, and Zamora, 2021 (Ferratges et al., Reference Ferratges, Artal and Zamora2021a); E. veteris n. sp.
Type material
The holotype, MGSB77593, is a complete left propodus (length: 31.0 mm; palm length: 24.0 mm; palm height: 19.0 mm) with well-preserved cuticle. There are two paratypes, MPZ 2021/29 and MPZ 2022/13, complete left propodi.

Figure 7. Eocalcinus veteris n. sp. (1–5) Paratype MPZ 2021/29), left chela: (1) lateral view of inner side; (2) frontal view; (3) lateral view of outer side; (4) upper view; (5) inferior view. (6, 7) Isolated dactylus (MPZ 2022/13) in lateral and occlusal margin (inferior view). (8–11) Holotype (MGSB77593), left chela: (8) oblique lateral view of outer side; (9) oblique frontal view; (10) upper view; (11) frontal view.

Figure 8. Shape change of the left chelas of Eocalcinus during the Eocene.
Diagnosis
Left propodus semicircular, stout. Palm longer than high; lower margin sinuous in both lateral and lower views. Fixed finger with occlusal edge sinuous, obliquely oriented.
Description
The complete propodus of the holotype is 32.0 by 20.0 mm. Palm only somewhat longer than high. Lower margin slightly sinuous, nearly straight in proximal portion, fixed finger curving downward. Lower margin less ridged, more rounded. Dense tiny granulation on outer surface and fingers. Granules close together, pavement-like. Spaced bigger granules in upper portion. Clear setal pits, mainly on fingers. Inner portion of palm smooth, with scarce granules (Fig. 7).
Stout left propodus planoconvex and subcircular. Lower margin sinuous in lateral and lower inferior views; outer surface convex; inner surface weakly convex, nearly flat. Palm slightly longer than high. Fixed finger short, robust, arched (strongly convex). Dactylus very robust, triangular in cross section, with the occlusal edge concave, smooth. Ornamentation on the fixed finger and palm is densely covered with small granules, very close together (pavement-like), and very uniform.
Etymology
The specific name veteris comes from Latin and means “old,” “ancient,” referring to the fact that it is the oldest member of the genus.
Other material examined
Thirty-six additional specimens numbered MGSB77594a–z and MGSB85959a–j and 24 additional specimens numbered MPZ 2022/14–37.
Remarks
The studied specimens can be assigned to Eocalcinus because of the general outline of the left chela, being hemicircular in shape; the lower margin of the propodus that is concave in the middle portion; the upper margin of the palm, which is broadly arched; the robust fixed finger, without teeth on the occlusal edge, joining tightly the movable finger; the dactylus, which exhibits a broadly arched upper margin; and because the whole chela is densely ornamented with small granules. The new species, E. veteris, is clearly distinguishable from other species of the genus in having a less subcircular general outline; the distinction is also based on a palm somewhat longer than high and a lower margin convex in the proximal portion and concave in the middle portion.
The type species, E. eocenicus, shows some differences from E. veteris n. sp. In E. eocenicus, the major chela is more semielliptical; the propodus longer than high, the palm longer than high; the lower margin of the propodus is nearly straight, only slightly concave in the middle portion; the fixed finger has straight margins; the dactylus is nearly straight in the occlusal edge and exhibits notable small teeth in the upper margin. The lower margin is only slightly sinuous from lateral view (slightly concave in median portion), strongly sinuous when seen from the lower view. The lower margin is strongly ridged and raised in the proximal portion (adaptation for gastropod apertures). There are spaced large granules, mainly in the upper portion; the ornamentation in the lower portion of the palm consists of very small circular granules, very uniformly distributed (Via, Reference Via1959), while granules are smaller in the new species. Granules are larger in E. eocenicus than in E. veteris n. sp. Inner portion of palm smooth, with scarce granules in E. veteris.
Eocalcinus cavus has a more elongated left chela; the palm is longer than high; the lower margin of the propodus is nearly straight and only weakly concave; the fixed finger is much more elongated, and the occlusal margin is only somewhat arched; the lower portion of the palm bears larger granules (Beschin et al., Reference Beschin, Busulini, De Angeli and Tessier2002; De Angeli and Caporiondo, Reference De Angeli and Caporiondo2017). Comparison of the new species with E. albus is almost impossible because the latter was described on the basis of a single dactylus only. However, this dactylus has a totally straight occlusal margin, and the upper margin is gently denticulated (Beschin et al., Reference Beschin, Busulini and Tessier2010).
All other species of Eocalcinus, with the exception of the type species, are represented by the left chelae (or a single dactylus of the left chelae in the case of E. albus). Recent finds of the right chelae of E. eocenicus allowed the assignment of this genus to the family Calcinidae (Ossó, Reference Ossó2020).
Eocalcinus veteris n. sp. corresponds to the stratigraphically oldest species of the genus (see Fig. 8) and allows us to trace a general trend toward more rounded shapes (Fig. 8). This oldest species presents a more elongated outline, a straighter lower margin, and a less marked plano-convex section (O-shaped section) than in more recent species (D-shaped section). This trend toward more rounded shapes, with a sinuous lower margin and a more plano-convex section, could be related to the progressive adaptation of the major chela to perform an opercular function, adapting to the shape of the aperture of the host shell (as proposed by Ferratges et al., Reference Ferratges, Artal and Zamora2021a, p. 9, figs. 4, 5).
Family Paguridae Latreille, Reference Latreille1802
Genus Pagurus Fabricius, Reference Fabricius1775
Type species
Cancer bernhardus Linnaeus, Reference Linnaeus1758 by original designation.
Fossil species included
Pagurus alabamensis Rathbun, Reference Rathbun1935; P. alatoides Philippe and Secrétan, Reference Philippe and Secretan1971; P. albus Müller, Reference Müller1979 (=P. tuberculosus Harvey, Reference Harvey1998); P. avellanedai Via, Reference Via1951; P. banderensis Rathbun, Reference Rathbun1935; p. aff. P. bernhardus (Linnaeus, Reference Linnaeus1758); P. concavus Müller, Reference Müller1979; P. convexus Whetstone and Collins, Reference Whetstone and Collins1982; P. granosipalm (Stimpson, Reference Stimpson1859); P. langei Collins and Jakobsen, Reference Collins and Jakobsen2003; P. latidactylus Müller and Collins, Reference Müller and Collins1991; P. malloryi Schweitzer and Feldmann, Reference Schweitzer and Feldmann2001; P. manzonii (Ristori, Reference Ristori1888); P. marceti Via, Reference Via1959; P. marini Via, Reference Via1959; P. mezi Lörenthey, Reference Lőrenthey1909; P. rakosensis Müller, Reference Müller1979; P. squamosus Ristori, Reference Ristori1886; P. texensis Franţescu, Reference Franţescu2014; P. travisensis Stenzel, Reference Stenzel1945; P. turcus Müller, Reference Müller1984, and P. valdagnensis Beschin et al., Reference Beschin, De Angeli, Checchi and Zarantonello2012.
?Pagurus sp.
Figure 9
Description
Right cheliped moderately stout, short (Fig. 9). Palm subquadrate in shape, densely covered by subconical granules on the outer surface; inner surface smooth; gently convex dorsal surface, with numerous closely spaced small conical tubercles; inner surface gently convex, with scattered small, low tubercles. Lower margin slightly concave. The preserved portion of dactylus robust, with the same ornamentation as the palm.

Figure 9. ?Pagurus sp. (1–3) Right cheliped (specimen MPZ 2021/32): (1) lateral view of outer side; (2) lateral view of inner side of right chela; (3) oblique upper view. Specimen whitened with ammonium chloride sublimate.
Material
MPZ 2021/32 is a partial right chela (length 6.2 mm and width 6.5 mm) with well-preserved cuticle, and MPZ 2022/60 is a partial right propodus.
Remarks
Numerous fossil taxa have been assigned to the genus Pagurus (see the preceding), and it is widely acknowledged to most likely be a cluster of different genera, so a revision is necessary (Schweitzer and Feldmann, Reference Schweitzer and Feldmann2001). This happens because most of the taxonomic and diagnostic features to differentiate between modern genera are not preserved in fossil material (i.e., Jagt et al., Reference Jagt, van Bakel, Fraaije and Neumann2006; Fraaije, Reference Fraaije2014; Fraaije et al., Reference Fraaije, Krzemiński, van Bakel, Krzemińska and Jagt2014).
The recovered material consists of a very incomplete single right chela, but it shares several features with extant members of Pagurus. According to the general shape of the chela (a well-developed palm that maintains its height along its entire length) and dense tuberculate ornamentation, appears to be most similar to the genus Pagurus. For this reason, we have taken the most conservative approach and placed the material within Pagurus.
The extant species of the genus present a robust right chela, globular, with outer and inner surface of the palm strongly convex, and the outer surface of propodus covered by dense granules (Sánchez and Campos, Reference Sánchez and Campos1978; McLaughlin et al., Reference McLaughlin, Komai, Lemaitre and Rahayu2010; Lima and Lemaitre, Reference Lima and Lemaitre2016). Fossil species are also characterized by globular, convex surfaces with the outer surface of the propodus densely granulated (Via, Reference Via1959; Schweitzer and Feldmann, Reference Schweitzer and Feldmann2001; De Angeli and Caporiondo, Reference De Angeli and Caporiondo2017). Some recent species (i.e., P. spinossior Komai, Reshmi, and Kumar, Reference Komai, Reshmi and Kumar2013) bear similarities with the scarce material recovered, so we tentatively assign the new material to this genus.
Genus Anisopagurus McLaughlin, Reference McLaughlin1981
Type species
Pylopagurus bartletti Milne Edwards and Bouvier, Reference Milne Edwards and Bouvier1893 by subsequent designation of McLaughlin, Reference McLaughlin1981.
Species included
Anisopagurus actinophorus Lemaitre and McLaughlin, Reference Lemaitre and McLaughlin1996; A. asteriscus Lemaitre, Reference Lemaitre2020; A. bartletti (Milne Edwards and Bouvier, Reference Milne Edwards and Bouvier1893); A. hopkinsi Lemaitre and McLaughlin, Reference Lemaitre and McLaughlin1996; A. pygmaeus (Bouvier, Reference Bouvier1918); A. vossi Lemaitre and McLaughlin, Reference Lemaitre and McLaughlin1996.
Remarks
The genus Paguritta Melin, Reference McLaughlin, Komai, Lemaitre and Rahayu1939 shows similarities with Anisopagurus due to the general shape of the chela and the row of spines on the upper and lower margins of the palm. Anisopagurus is distinguishable from Paguritta in having the outer surface of the palm densely covered by hemispherical, pearled granules closely spaced (while all species of Paguritta bear small conical spines); the fingers are characterized by strong longitudinal ridges, while in all species of Paguritta the fingers are flattened (see Komai and Nishi, Reference Komai and Nishi1996, p. 463–464, 472, figs. 4, 5; Komai and Okuno, Reference Komai and Okuno2001, p. 299, figs. 3, 4; McLaughlin and Lemaitre, Reference McLaughlin and Lemaitre1993, p. 5, figs. 1, 3, 5, 7, 9, 11).
The modern genus Rhodochirus McLaughlin, Reference McLaughlin1981 also shows similarities with Anisopagurus in the general shape of the chela. However, Rhodochirus presents some differences, such as the more pointed fingers, coalescent granules on fixed finger, outer surface of the palm covered with large spiny tubercles with basal rosettes (see McLaughlin, Reference McLaughlin2003, p. 117, 127, fig. 6; Parente and Hendrickx, Reference Parente and Hendrickx2005, fig. 1; Komai, Reference Komai2013, p. 29).
Anisopagurus primigenius new species
Figure 10
Type material
The holotype, MPZ 2021/31, is a complete right propodus (propodus length: 9.9 mm; palm length: 5.2 mm; palm height: 5.6 mm) with well-preserved cuticle but without movable finger. There are two incomplete right propodi (paratypes), MPZ 2022/39 and MGSB77624.

Figure 10. Anisopagurus primigenius n. sp. (1–4) Holotype (MPZ 2021/31), right chela: (1) lateral view of outer side; (2) oblique upper view; (3) frontal view. (4) Detail of the occlusal margin with two teeth. (5–7) Paratype (MGSB77624), right chela: (5) lateral view of outer side; (6) frontal view; (7) lateral view of inner side. Specimens whitened with ammonium chloride sublimate.
Diagnosis
Right cheliped suboperculate, D-shaped in cross section; posterior margin slightly offset toward the inner surface; outer surface of palm tuberculated, surrounded by spines directed vertically; inner surface with squamous tubercles.
Description
Right cheliped suboperculate (Fig. 10), ovate, approximately twice as long as high, flattened dorsoventrally, D-shaped in cross section; angle of articulation propodus/carpus 15° from perpendicular; upper margin broadly arched, bearing small conical teeth directed forward; lower margin slightly arched. Palm semicircular, as long as high, with median region moderately elevated in the outer surface, surrounded by a more or less flat surface. Outer surface covered with numerous fungiform tubercles and surrounded by strong spines directed nearly vertically forming crown-like shape (Fig. 10). Inner surface convex, with small squamous tubercles. Fingers slender and elongated, dactylus and fixed finger with a longitudinal ridge. Fixed finger with blunt termination, about as long as the palm. Occlusal margin with two aligned molariform teeth. Left cheliped unknown.
Etymology
From the Latin adjective primigenius (the oldest) to emphasize the geological seniority of this paguroid.
Other material examined
Two partial right propodi (MPZ 2022/39 and MPZ 2022/61) and one isolated right dactylus (MPZ 2022/40).
Remarks
Ferratges et al. (Reference Ferratges, Zamora and Aurell2021b) tentatively suggested that this taxon could be assigned to either Paguritta Melin, Reference Melin1939 or Rhodochirus McLaughlin, Reference McLaughlin1981. However, a more detailed study of the material suggests that this species fits better in Anisopagurus. Anisopagurus primigenius n. sp. can be differentiated from other species of the genus on the basis of its density of ornamentation and shape of its fungiform tubercles, covering the entire outer surface, very tight on both fingers, almost coalescing, and the two rows of spines on the upper margin.
A. primigenius n. sp. is morphologically close to species of Paguritta due to the general shape of the chela and the row of spines in the upper and lower margins of the palm. Nevertheless, A. primigenius n. sp. is easily distinguishable from Paguritta sp. by having the outer surface of the palm densely covered by closely spaced hemispherical, pearled granules (while all species of Paguritta bear small conical spines); the fingers are characterized by strong longitudinal ridges in A. primigenius n. sp. while in all species of Paguritta the fingers are flattened (see Mclaughlin and Lemaitre, Reference McLaughlin and Lemaitre1993, p. 5, figs. 1, 3, 5, 7, 9, 11; Komai and Nishi, Reference Komai and Nishi1996, p. 463–464, 472, figs. 4, 5; Komai and Okuno, Reference Komai and Okuno2001, p. 299, figs. 3, 4).
The modern species assigned to Rhodochirus also show similarities with Anisopagurus primigenius n. sp. in the general shape of the chela. However, differences include the more pointed fingers, coalescent granules on the fixed finger, and outer surface of the palm covered with large spiny tubercles with basal rosettes (see McLaughlin, Reference McLaughlin2003, p. 117, 127, fig. 6; Parente and Hendrickx, Reference Parente and Hendrickx2005, fig. 1; Komai, Reference Komai2013, p. 29).
Regarding the fossil record, Anisopagurus primigenius n. sp. seems to share some characteristics with Lessinipagurus granulatus and L. planus, such as the general ornamentation and the fixed and movable fingers with elongated longitudinal ridges. Nevertheless, A. primigenius n. sp. presents differences in the general shape, with a more elongated propodus and fingers. In Lessinipagurus, the complete chela is subcircular, not elongated, and the upper margin is extremely salient, visor-shaped (see Beschin et al., Reference Beschin, De Angeli, Checchi and Zarantonello2012, p. 29, fig. 22; De Angeli and Caporiondo, Reference De Angeli and Caporiondo2017, p. 20–22, figs. 14, 15).
Final remarks and conclusions
The global record of Paleogene paguroids is poor and often fragmentary. Specifically, in the Iberian Peninsula, only six species have previously been described from the Eocene. Via (Reference Via1959) first described Pagurus marceti, Pagurus marini, and Eocalcinus eocenicus on the basis of fragmentary material. Ferratges et al. (Reference Ferratges, Zamora and Aurell2020) described a nearly complete specimen of Diogenes augustinus, and Ferratges et al. (Reference Ferratges, Zamora and Aurell2021b) recently described two new species (Parapetrochirus robustus and Eocalcinus gerardbretoni) from the lower and upper Eocene, respectively.
This new contribution includes representatives of four families (Annuntidiogenidae, Diogenidae, Calcinidae, and Paguridae) and increases our knowledge of known taxa (six new species) of paguroids from the early Eocene associated with reef environments. Specifically, the studied assemblage of paguroids inhabited shallow reef complexes of the Serraduy Formation within the euphotic to mesophotic zone (see Ferratges et al., Reference Ferratges, Zamora and Aurell2021b).
Some of the taxa studied in the present work show close relationships with several modern genera (Anisopagurus, Clibanarius, Dardanus, Paguristes, Pagurus, Petrochirus). In general, these modern hermit crabs are common in intertidal and shallow-water areas of tropical and temperate seas (i.e., Forest and Saint-Laurent, Reference Forest and de Saint-Laurent1968; Hazlett, Reference Hazlett1981; Leite et al., Reference Leite, Turra and Gandolfi1998; Melo, Reference Melo1999; Rahayu, Reference Rahayu2006; Rahayu and Forest, Reference Rahayu and Forest2009; Mantelatto et al., Reference Mantelatto, Fernandes-Góes, Fantucci, Biagi, Pardo and de Goes2010; McLaughlin et al., Reference McLaughlin, Komai, Lemaitre and Rahayu2010; Trivedi and Vachhrajani, Reference Trivedi and Vachhrajani2017).
This study contributes to the understanding of paguroid diversity during the Eocene in the southern Pyrenean basins. In addition, the data provided increase the knowledge of European fossil paguroids, providing several new taxa, some of which correspond to the oldest representatives of their respective genera. Our study also increases the temporal distribution of the genus Eocalcinus with the oldest record of the genus. In addition, the new materials assigned to this genus suggest an evolutionary trend toward more rounded shapes. The oldest species of Eocalcinus had a more elongated outline, a straighter lower margin, and a less marked plano-convex section of the palm. This trend toward more rounded shapes could be related to the progressive adaptation of the major chela to perform an opercular function (Ferratges et al., Reference Ferratges, Artal and Zamora2021a).
Gastropod shells are vital for most hermit crab species, being essential for their survival (see Tricarico and Gherardi, Reference Tricarico and Gherardi2006 and references therein). The great abundance and diversity of gastropods observed in the studied outcrop (see Ferratges et al., Reference Ferratges, Zamora and Aurell2021b) probably contributed to the diversity of hermit crabs. In modern ecosystems, the availability of gastropod shells plays an important role in limiting the abundance of hermit crabs (Vance, Reference Vance1972; Bach et al., Reference Bach, Hazlett and Rittschof1976; Kellogg, Reference Kellogg1976), and their diversity reduces competition between different genera. In fact, some modern species show a marked preference for certain empty shells over others (i.e., Vance, Reference Vance1972; Conover, Reference Conover1978; Bertness, Reference Bertness1980). Thus, the mechanism allowing coexistence of several taxa in the same environment involves both resource and habitat partitioning (Vance, Reference Vance1972).
The data provided here show a great diversity of paguriods at the beginning of the Eocene, which is richer than Paleocene records (see Jakobsen et al., Reference Jakobsen, Fraaije, Jagt and van Bakel2020 and references therein), and show that the reefs of the lower Eocene were important hotspots of pagurid diversities comparable to modern ecosystems.
Acknowledgments
This work has been supported by the project CGL2017-85038-P subsidized by the Spanish Ministry of Science and Innovation, the European Regional Development Fund, and Project E18-20R Aragosaurus: Recursos Geológicos y Paleoambientes of the government of Aragón-FEDER. The research of F.A. Ferratges is funded by an FPU Grant (Spanish Ministry of Science and Innovation). I. Pérez provided photographic assistance. The staff of the MGSB allowed the study of their historical decapod collections. We are also grateful to the reviewers C.E. Schweitzer (Kent State University) and R.H.B. Fraaije (Oertijdmuseum Boxtel, the Netherlands), who greatly improved the resulting manuscript.













