Introduction
Globally, the ichnology of freshwater settings has received limited attention in comparison with their marine counterparts (Buatois and Mángano, Reference Buatois, Mángano and McIlroy2004; Hasiotis, Reference Hasiotis2004; MacEachern et al., Reference MacEachern, Bann, Pemberton, Gingras, MacEachern, Bann, Gingras and Pemberton2007; Melchor et al., Reference Melchor, Genise, Buatois, Umazano, Knaust and Bromley2012; Genise, Reference Genise2017; Savrda, Reference Savrda2019). The Turonian Ferron Sandstone of the “Last Chance” depocenter in central Utah, USA, is a siliciclastic succession composed of fluvial-deltaic sandstone, mudstone, and coal (Ryer et al., Reference Ryer, Phillips, Bohor and Pollastro1980; Garrison and van den Bergh, Reference Garrison, van den Bergh, Chidsey, Adams and Morris2004; Ryer, Reference Ryer, Chidsey, Adams and Morris2004) with extensive outcrops for examining ichnology in continental and marine realms. There have been many reports of the invertebrate ichnology in marginal marine strata within the upper Ferron Sandstone of this area (Ryer, Reference Ryer1981; Anderson and Ryer, Reference Anderson, Ryer, Chidsey, Adams and Morris2004; Bhattacharya and Davies, Reference Bhattacharya, Davies, Chidsey, Adams and Morris2004; Corbeanu et al., Reference Corbeanu, Wizevich, Bhattacharya, Zeng, McMechan, Chidsey, Adams and Morris2004; Gardner et al., Reference Gardner, Cross, Levorsen, Chidsey, Adams and Morris2004; Garrison and van den Bergh, Reference Garrison, van den Bergh, Chidsey, Adams and Morris2004; Moiola et al., Reference Moiola, Welton, Wagner, Fearn, Farrell, Enrico, Echols, Chidsey, Adams and Morris2004; Ryer and Anderson, Reference Ryer, Anderson, Chidsey, Adams and Morris2004; Gani et al., Reference Gani, Bhattacharya, MacEachern, MacEachern, Bann, Gingras and Pemberton2007; Bhattacharya and MacEachern, Reference Bhattacharya and MacEachern2009), yet continental traces are seldom described in these studies because of their focus related on nearshore geobodies as potential reservoir analogs. However, King and Anderson (Reference King, Anderson, Morris and Ressetar2013) described in situ plant traces and probable insect traces (Haplotichnus [now Treptichnus; Getty and Bush, Reference Getty and Bush2017] and trace fossils with meniscate and pustulose textures) and King et al. (Reference King, Botterill, Gingras and Pemberton2020) described continental traces attributed to mollusk locomotion (Archaeonassa) and potentially mayflies (Rhizocorallium exhibiting spreite) from the Ferron Sandstone. This study provides examples of new trace fossil morphologies attributable to filter-feeding subaqueous insects (e.g., mayfly larvae) in freshwater channel deposits. These can be used for paleoenvironmental reconstruction in deposits from the Mesozoic to present. Additionally, these distinctive burrows can be used to evaluate colonization trends over time (e.g., substrate preference, paleoclimate, channel size, river style, flow regimes) that can tell us more about the adaptation and evolution of subaqueous insects as infaunal filter feeders.
Geological setting
The slab containing the holotype and syntypes (NCSM 12618) and the other paratype slabs (NCSM 12619–NCSM 12625) were discovered within coastal plain deposits of the Ferron Sandstone in Hidden Valley, Utah. The “axiotype” (NCSM 12626) (sensu Lucas and Harris, Reference Lucas and Harris2020) was collected 4.33 km away, just north of Cowboy Canyon (Figs. 1, 2). Lupton (Reference Lupton1916) named the coals of the upper Ferron Sandstone at the Last Chance area in ascending order, using letters that proved instrumental for Ryer (Reference Ryer1981), who built the initial stratigraphy of the Ferron Sandstone in this area. The Hidden Valley site lies between the “C” and “G” coal zones, and the Cowboy Canyon site is situated between the “G” and “I” coal zones (Figs. 2, 3). The two surfaces where specimens were collected (fallen block in Hidden Valley) or molded (in situ at Cowboy Canyon) are from the bases of small (<6 m thick, <320 m wide) channel sandstones (Fig. 3) overlying platy, blocky gray mudstone/silty mudstones in the respective areas. King et al. (Reference King, Botterill, Gingras and MacEachern2020b) detailed the sedimentology and stratigraphy of the strata containing these trace fossils in the Cowboy Canyon area showing that the channels are encased in coastal plain sediment, and that the Glossifungites colonization surfaces occur at multiple stratigraphic levels, which suggests a link with autogenic processes rather than the allogenic surfaces that Glossifungites (i.e., G. saxicava Łomnicki, Reference Łomnicki1886) are normally associated.

Figure 1. Location map indicating where the type specimens of Glossifungites gingrasi n. isp. were collected in Hidden Valley (yellow star), and where the axiotype was collected at the mouth of Cowboy Canyon (orange star) (modified from Anderson et al., Reference Anderson, McClure, Chidsey, Ryer and Morris2003; Garrison and van den Bergh, Reference Garrison, van den Bergh, Chidsey, Adams and Morris2004). Surficial units: Blue Gate Shale (light gray), Ferron Sandstone (beige), Tununk Shale (dark gray), Quaternary alluvium (white). Red line indicates a contemporaneous stratigraphic surface to the surface from which the axiotype was collected, which contains numerous additional examples of the ichnofossil.
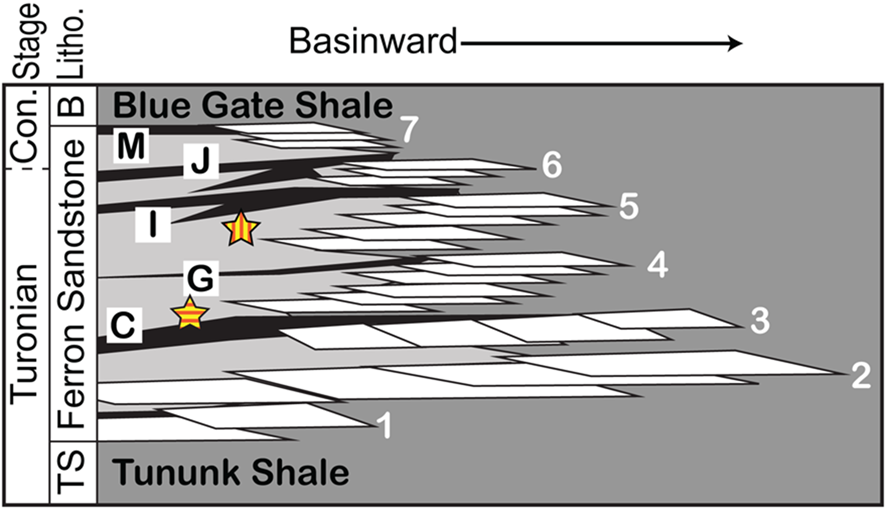
Figure 2. Schematic of the Ferron Sandstone stratigraphy in the study area. Stars indicate the stratigraphic levels where Glossifungites gingrasi n. isp. type specimens (Hidden Valley; star with horizontal stripes) and axiotype (Cowboy Canyon; star with vertical stripes) were collected (modified from Ryer et al., Reference Ryer, Phillips, Bohor and Pollastro1980; Ryer, Reference Ryer1981; Gardner et al., Reference Gardner, Barton, Tyler, Fisher and Flores1992). The numbers represent parasequence sets and letters represent coal zones. Con. = Coniacian, Litho. = Lithostratigraphic Nomenclature.
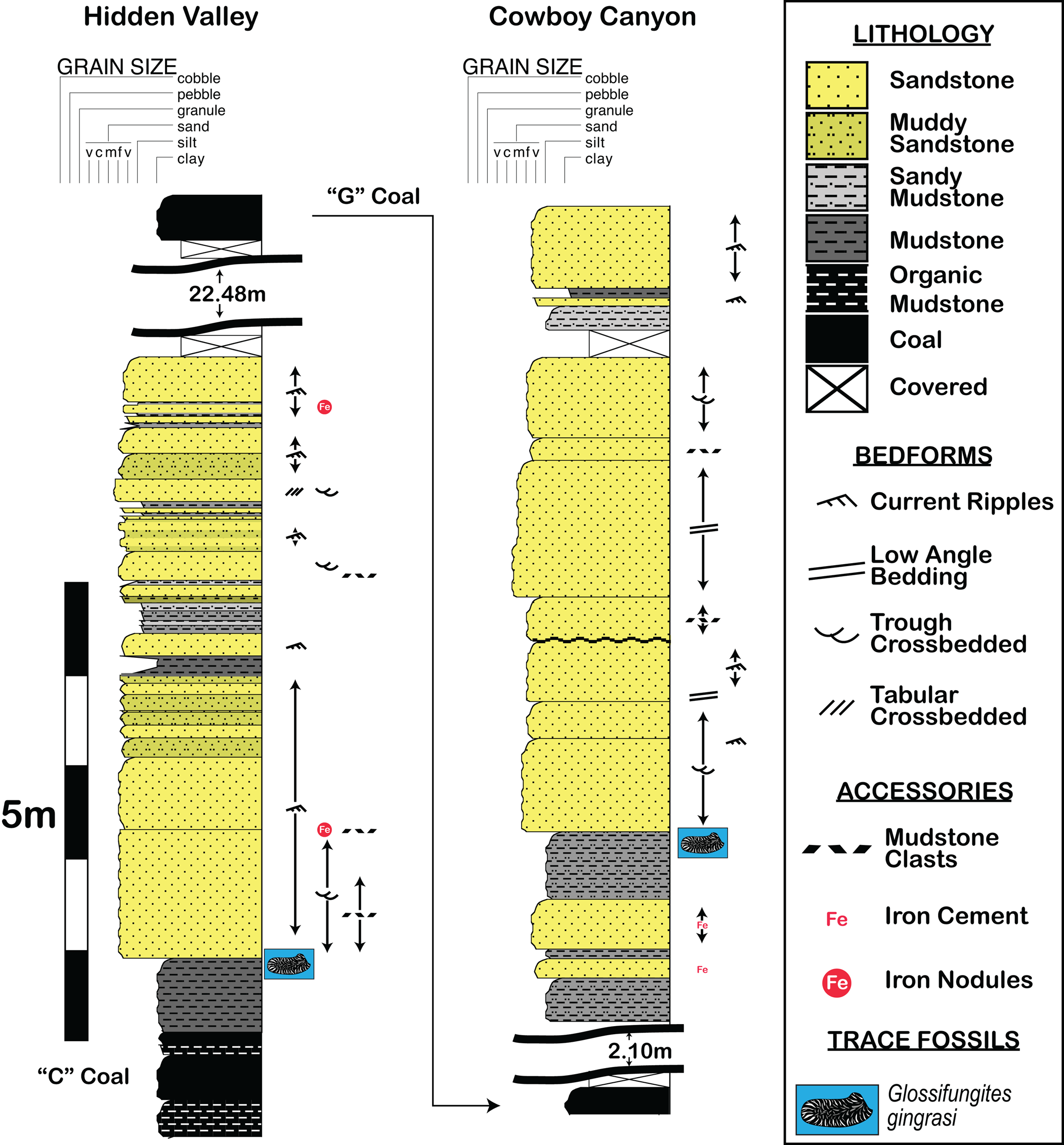
Figure 3. Stratigraphic logs showing the position of Glossifungites gingrasi n. isp. in Hidden Valley (over mudstone above the “C” Coal zone), and in Cowboy Canyon (above mudstone of the “G” Coal zone).
Repository and institutional abbreviation
The holotype, syntypes, paratypes, and axiotype examined in this study are deposited at North Carolina Museum of Natural Sciences (NCSM) in Raleigh, North Carolina, USA.
Systematic ichnology
Ichnogenus Glossifungites Łomnicki, Reference Łomnicki1886
Type ichnospecies
Glossifungites saxicava Łomnicki, Reference Łomnicki1886 from the Miocene deposits of the Lviv region, Ukraine.
Emended diagnosis
Passively filled, horizontal to oblique, unbranched, tongue-shaped burrows with a medial area more depressed or narrower than the lateral area. The distal portion of the trace fossil is usually wider than the apertural portion. The surface of the trace fossil is covered by bioglyphs (adapted from Łomnicki, Reference Łomnicki1886 and Belaústegui et al., Reference Belaústegui, Ekdale, Domènech and Martinell2016a).
Remarks
Since the initial definition of the tongue-shaped, scratch-marked trace fossil Glossifungites, there have been several attempts to reassign the ichnogenus as a junior synonym of other forms. The convoluted ichnotaxonomic history of the trace fossil has been detailed by Uchman et al. (Reference Uchman, Bubniak and Bubniak2000), who argued ichnotaxonomy should follow Fürsich (Reference Fürsich1974), placing Glossifungites saxicava as a junior synonym of Rhizocorallium jenense Zenker, Reference Zenker1836. The work of Uchman et al. (Reference Uchman, Bubniak and Bubniak2000) was based on the fact that R. jenense showed both scratch-marked and non-scratch-marked specimens within the same stratal layer, demonstrating a continuum of characteristics. Fürsich (Reference Fürsich1974) differentiated Rhizocorallium ichnospecies based on overall length and pattern rather than using differentiation of the fill as an ichnotaxobase, and did not adequately detail why Glossifungites was a junior synonym of Rhizocorallium. More recently, Knaust (Reference Knaust2013) concluded that there are only two valid ichnospecies of Rhizocorallium (R. jenense and R. commune Schmid, Reference Schmid1876), and following Fürsich (Reference Fürsich1974), included Glossifungites under Rhizocorallium, although the two papers are not in complete agreement on the classification with regards to the importance of spreite versus passive fill or ichnotaxobase hierarchy (ichnospecies versus ichnosubspecies and variety). More recently, Belaústegui et al. (Reference Belaústegui, Ekdale, Domènech and Martinell2016a) refuted that Glossifungites was a junior synonym of Rhizocorallium, and reintroduced Glossifungites saxicava as a valid ichnospecies using examples from Spain that contained barnacle-colonized interiors to illustrate the importance of differentiating passive versus active fills and the presence/absence of spreite as ichnotaxobases for differentiating ichnogenera. The passive infilling and interior barnacle colonization in Belaústegui et al. (Reference Belaústegui, Ekdale, Domènech and Martinell2016a) provided evidence that Glossifungites is a distinctive tongue-shaped structure and does not belong in the same ichnogenera as trace fossils resulting from a migrating, tubular, U-shaped structure, which defines Rhizocorallium. Here, we follow the most recent nomenclature (Belaústegui et al., Reference Belaústegui, Ekdale, Domènech and Martinell2016a) in referring tongue-shaped, passively filled specimens to the ichnogenus Glossifungites.
Glossifungites gingrasi new ichnospecies
Figures 4–7, 8.4–8.7

Figure 4. The holotype (arrow) of Glossifungites gingrasi n. isp. exhibiting transverse radiating scratch marks (bioglyphs). Inset (upper right) diagrammatic location of holotype (H) and examples of syntypes (S) on slab specimen (NCSM 12618). Note the weathering of mudstone clasts, indicating a passive fill, which is common in distal portions of specimens. Photo taken in the field after removal.

Figure 5. Selected Glossifungites gingrasi n. isp. from (9) holotype (NCSM 12618), syntypes (NCSM 12618: 3, 4, 7, 13), and paratypes (NCSM 12619: 14; NCSM 12620: 12, 15; NCSM 12621: 2; NCSM 12622: 5, 6, 8; NCSM 12623: 11; NCSM 12624: 1; NCSM 12625: 10) showing the underside of the traces with bioglyphs that are perpendicular to the margin distally, but may roughly parallel the axis in the medial proximal area. Scale is 3 cm (bottom left) for 1-15. In limited side preservation (NCSM 12623: 16; NCSM 12618: 17), bioglyphs descend distally. Scale for 16, 17 is 1 cm.
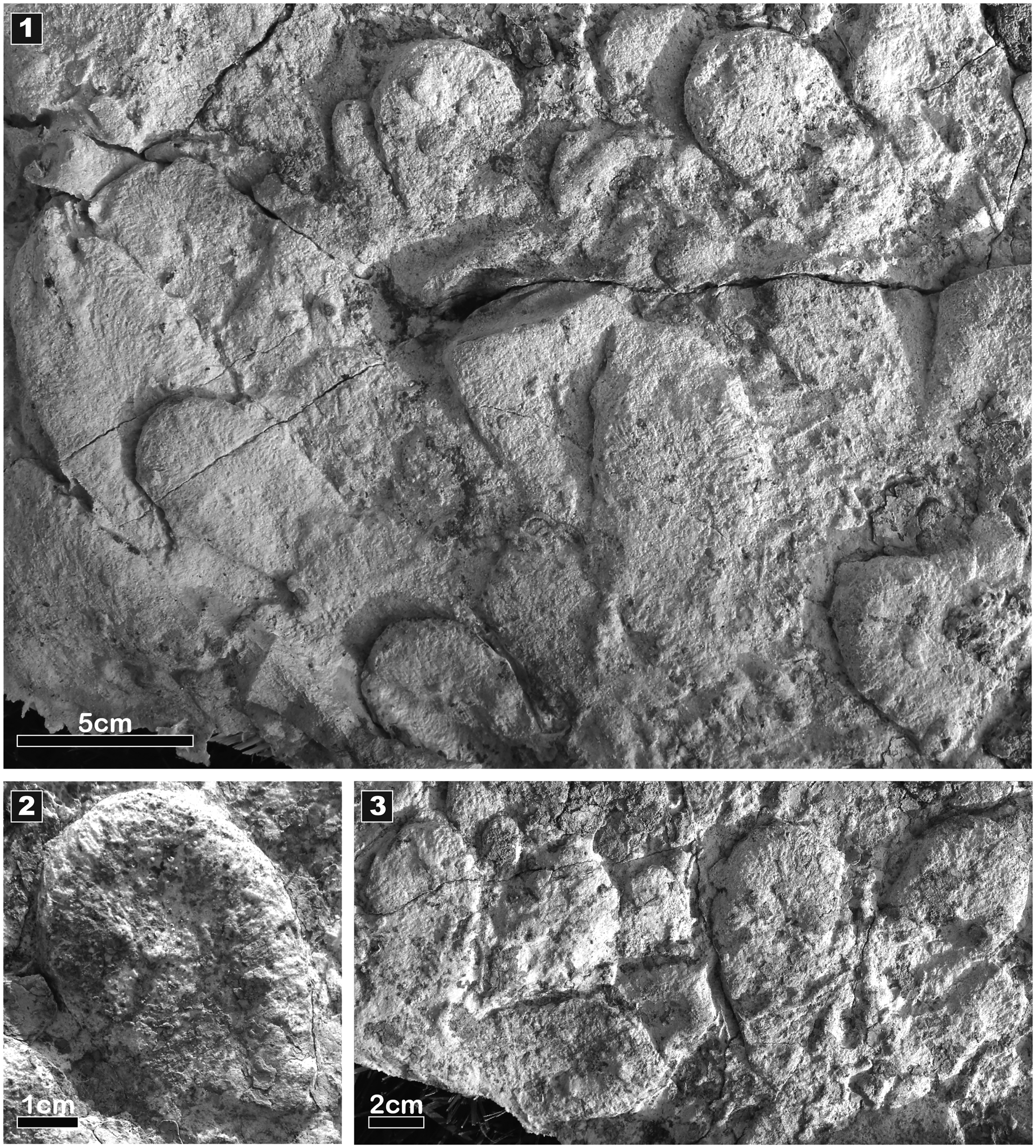
Figure 6. Selected photos of Glossifungites gingrasi n. isp. from the axiotype (NCSM 12626) in the Cowboy Canyon Area showing the same features as seen in the Hidden Valley type specimens, such as an overall tongue shape with medial axial bioglyphs (1) and transverse bioglyphs along margins with a radiating appearance and depressed central area more prevalent in longer forms (2, 3).

Figure 7. An outcrop photo on the underside of the sandstone in Cowboy Canyon to the left of where the axiotype was molded, showing the often roughly unidirectional nature of Glossifungites gingrasi n. isp., as well as examples of multiple small variations in the trace. These small variations include distal widening (white arrows) that may be associated with growth (ichnogeny). This surface also presents evidence that these traces may be much longer (black arrow) than the type specimens, but the surface is unreachable to discount the specimen being a composite trace.

Figure 8. Comparison of Glossifungites saxicava, G. gingrasi n. isp., and modern mayfly burrows. (1) Illustration of type specimen of Glossifungites saxicava (modified from Łomnicki, Reference Łomnicki1886); (2, 3) examples of G. saxicava (modified from Belaústegui et al., Reference Belaústegui, Ekdale, Domènech and Martinell2016a).; note the bioglyphs in G. saxicava may be in a crisscrossed, net-like pattern, but bioglyphs generally parallel the causative tube, and are typically transverse between the arms of the trace. Conversely, G. gingrasi n. isp. ([4] silicone mold of holotype, [5] holotype, [6] specimen from outcrop in Cowboy Canyon, [7] silicone mold of a syntype, [8] illustration of [5], [9] illustration of [6]) shows roughly transverse bioglyphs to the causative tubes (black lines 8, 9), which can crisscross (orange lines 8, 9) and may exhibit either short transverse or axial (red lines 8, 9) bioglyphs in the medial portion of the trace fossil. These patterns in G. gingrasi n. isp. are similar to modern mayfly burrows (10, 11; modified from Uchman et al., Reference Uchman, Mikuláš and Stachacz2017) dominated by transverse bioglyphs (grayscale), some crisscrossing bioglyphs (orange), with some axial parallel scratches in the median (red) that can appear long and sweeping (potential composite bioglyphs). All scales represent 1 cm, with a single scale present for the multiple examples of the two ichnospecies, and blacked-out portions represent missing parts of the burrow or fossil.
Holotype
North Carolina Museum of Natural Sciences (Raleigh, North Carolina) specimen slab NCSM 12618 (45 x 37 cm) containing the holotype and at least 20 syntypes (Fig. 4). All physical specimens were collected from Hidden Valley using a TS 500i STIHL Cutquick rock saw, with the holotype cradled in plaster and burlap for transportation.
Paratypes
Small slab specimens (NCSM 12619–NCSM 12625; Fig. 5) and large (98 x 64 cm) axiotype slab mold (NCSM 12626; Fig. 6) housed at the North Carolina Museum of Natural Sciences (Raleigh, North Carolina). The axiotype was molded using high solids latex (~75%) ingrained with burlap for rigidity.
Diagnosis
Passively filled, horizontal to oblique, unbranched, tongue-shaped burrows with a medial area more depressed or narrower than the outer portion. Bioglyphs cover the trace, commonly crisscrossing, with lateral and distal bioglyphs exhibiting roughly transverse orientation. Medial bioglyphs may be oriented roughly parallel to the axis, in places giving the bioglyphs an overall radiating or feather-like appearance.
Occurrence
All specimens were collected from the Turonian Ferron Sandstone. The holotype and paratype specimens were collected from a displaced sandstone block within Hidden Valley. Specimens were collected from the base of the sandstone block in slabs of various sizes. The occurrence of these trace fossils in Hidden Valley is sparse and localized. Alternatively, the Cowboy Canyon area, where the axiotype (plastotype) was collected, displays more prolific outcrop examples of these trace fossils. However, well-preserved specimens are exposed on the underside of sandstones ranging from 2.5–4.9 m above the ground, which makes acquisition of quality specimens problematic (other than taking molds and photographs).
Description
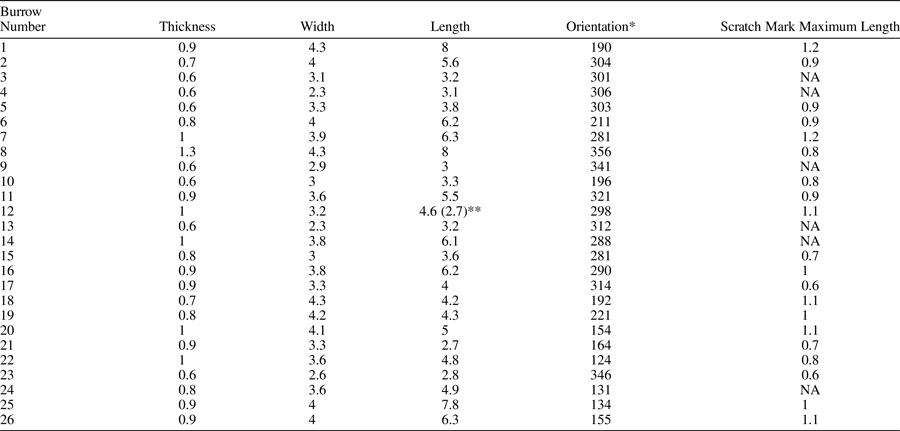
Preserved as convex hyporeliefs at the base of sandstone beds, with only the most distal portions ever exhibiting full relief. The trace fossils are passively filled by fine to medium sand grains and by gray mudstone clasts. Mudstone clasts appear to accumulate dominantly in the most distal portions of the trace when present (Figs. 4, 5.12, 5.14, 5.15), and commonly result in incomplete preservation or selective breakage of the trace (Figs. 4, 5.5, 5.8, 5.10). Trace fossils from the type block surface have maximum widths of 2.3–4.3 cm, lengths of 2.7–8 cm, and thicknesses (perpendicular to width) of 0.6–1.3 cm (Appendix). Dimensions of G. gingrasi n. isp. on the type surface (Appendix) are similar to examples of extant mayfly burrows from the Ohře River (Uchman et al., Reference Uchman, Mikuláš and Stachacz2017), which have widths of 2.0–4.0 cm, lengths of 4.6–8.0 cm, marginal tunnel diameters of 0.8–1.2 cm, and bioglyphs typically <1.2 cm long. Glossifungites gingrasi n. isp. in the study area are larger widths (>5.9 cm; King et al., Reference King, Botterill, Gingras and MacEachern2020b) than extant mayfly burrows. King et al. (Reference King, Botterill, Gingras and MacEachern2020b) speculated that the larger size of Glossifungites gingrasi n. isp. might be attributed to larger tracemakers or a variant body plan in the Cretaceous, when compared to extant organisms that are affected by anthropogenic stresses.
Longer traces of G. gingrasi n. isp. appeared to have greater variation in width (Figs. 6, 7). When comprising a sharp distinct boundary (external morphology), the causative structure (marginal tunnel) ranges from 0.9 to 1.4 cm in diameter. The medial depression (inward narrowing of the trace) is in places subtle, which makes it difficult to capture photographically in smaller specimens, but it is readily apparent in the most proximal portions of the trace, and in longer forms can manifest as two prominent arms (Figs. 5.14, 5.15, 6, 7). Specimens of Glossifungites gingrasi n. isp. share a similar morphological continuum, from short to long forms, with specimens of Glossifungites saxicava. As noted by Uchman et al. (Reference Uchman, Bubniak and Bubniak2000), in the G. saxicava type area, the width of smaller specimens of G. saxicava is rather consistent across the axial length, whereas in larger forms, the trace widens distally. A similar disparity is present in G. saxicava specimens of Belaústegui et al. (Reference Belaústegui, Ekdale, Domènech and Martinell2016a). This covariation of size and shape is also observed in specimens of G. gingrasi n. isp. from Hidden Valley (Figs. 4, 5) and Cowboy Canyon (Fig. 6). Both display constant widths in short form versus distally expanded elongate specimens. Locally, length is extended by stacking of burrows, as denoted by a sudden change in bioglyphs and depth (Fig. 5.3, 5.11).
Bioglyphs (sensu Bromley et al., Reference Bromley, Pemberton and Rahmani1984; Bromley, Reference Bromley1996; Ekdale and Gibert, Reference Ekdale and Gibert2010) reach a maximum width of ~1 mm and maximum lengths of 0.6–1.2 cm (Appendix). The lateral areas of the trace have the bioglyphs oriented transverse to the causative marginal tunnel (Figs. 5–7), whereas the medial bioglyphs show a more axial orientation (Figs. 4, 5.5, 5.9, 5.12, 5.13, 6.1). Bioglyphs on the lateral margins are rarely preserved in the study area, but when observed (Fig. 5.16, 5.17) appear to descend distally at an angle close to 45° with respect to the bottom of the trace. Because bioglyphs provide valid ichnotaxonomic characters, Knaust (Reference Knaust2013) used bioglyph ornamentation to differentiate R. commune from R. jenense, and the number of bioglyphs in a set has been used to distinguish Gnathichnus pentax Bromley, Reference Bromley1975. Although bioglyphs with similar orientations are visible in G. gingrasi n. isp., we cannot identify a consistent number of bioglyphs in sets across the various traces, largely because of the overlap and high density. Bioglyphs appear to become “composite bioglyphs” of two or more bioglyphs that share a similar orientation and lie on approximately the same plane. However, despite the lack of set identification, the transverse orientation of the bioglyphs is a unique and repeatable ichnotaxobase in G. gingrasi n. isp. Glossifungites saxicava has longitudinally orientated bioglyphs in the causative tube area (lateral and distal parts of the trace) and transverse-oriented bioglyphs in the medial depressed area between the two arms of the trace fossil (Fig. 8.1–8.3). In contrast, the distal and lateral portions of G. gingrasi n. isp. are dominated by bioglyphs transverse to the causative tube, and the medial portion of the trace fossil may contain some bioglyphs that are more axially oriented and can have a long sweeping appearance (potentially composite bioglyphs), often initiating from more proximal portions of the trace fossil (Fig. 8.4–8.9). In G. gingrasi n. isp., bioglyphs are dominantly perpendicular to the causative tube, and although they may be crisscrossed, they still maintain this general transverse orientation. Conversely, in similarly shaped, incongruent traces, such as Fuersichnus striatus Buatois, Reference Buatois1995, Rhizocorallium jenense, or R. commune (Knaust, Reference Knaust2013), the rarely crossing bioglyphs parallel causative tube orientation.
Glossifungites gingrasi n. isp. are oriented in respect to horizontal at a <9° angle in the study area, and often are grouped together locally in similar geographic orientations (Fig. 8, Appendix). Even on sloped surfaces, G. gingrasi n. isp. maintains a roughly horizontal axis, although the relationship to the slope is tangential. Conversely, Glossifungites saxicava in the type area is observed at angles approaching horizontal, as well as vertical (Uchman et al., Reference Uchman, Bubniak and Bubniak2000). Glossifungites saxicava is commonly at random orientations in the type area (Uchman et al., Reference Uchman, Bubniak and Bubniak2000), whereas Glossifungites gingrasi n. isp. in high densities often have a roughly unidirectional orientation (Appendix; Fig. 7). Traces may be crosscut by convex hyporeliefs of Palaeophycus <0.5 cm in diameter (Figs. 4, 5.10, 5.12, 5.15); however, that appears to be the extent of ichnodiversity on these surfaces.
Etymology
Named for Professor Murray K. Gingras (University of Alberta, Edmonton, Canada) who has greatly contributed to the ichnological community by unravelling the complexities of burrows in variable substrates and educating a new generation of ichnologists.
Materials
The slab NCSM 12618 contains the holotype and at least 20 syntypes, and NCSM 12619–12625 slabs have at least 36 individual G. gingrasi n. isp. trace fossils (paratypes), whereas the axiotype (NCSM 12626) has >68 individual examples. The outcrop in the Cowboy Canyon area has several hundred individuals in the sandstone overhangs.
Remarks
In examining the structure of modern mayfly burrows, Uchman et al. (Reference Uchman, Mikuláš and Stachacz2017) noted that the known mayfly-associated ichnotaxa (i.e., Asthenopodichnium, Arenicolites, Fuersichnus, Rhizocorallium jenense) do not apply to the tongue-shaped, scratch marked morphologies seen in the modern, and that there is room to establish new ichnotaxon. Provided herein is evidence in the form of a bioglyph orientation ichnotaxobase that is more consistent with modern examples than those of any ancient examples described so far. Abel (Reference Abel1935) described similar modern burrows as Ephemerites, but Fürsich and Mayr (Reference Fürsich and Mayr1981) argued that Ephemerites was a junior synonym of Rhizocorallium Zenker, Reference Zenker1836, and Knaust (Reference Knaust2013) declared Ephemerites as nomen nudum based on lack of a type specimen. Uchman et al. (Reference Uchman, Mikuláš and Stachacz2017) agreed with Knaust (Reference Knaust2013) and added that modern burrows should not provide the example for fossilized ichnotaxa nomenclature. Furthermore, the trace fossils described herein fit within the description of Glossifungites.
Discussion
Potential tracemakers
Tracemakers for tongue- and U-shaped burrows such as Glossifungites and Rhizocorallium (i.e., R. jenense), respectively, are generally considered to have been made by crustaceans, amphipods, polychaetes, and subaqueous insects that utilize these domiciles for filter-feeding (Seilacher, Reference Seilacher1967, Reference Seilacher2007; Fürsich, Reference Fürsich1974; Fürsich and Mayr, Reference Fürsich and Mayr1981; Knaust, Reference Knaust2013). However, detailed descriptions of burrows constructed by extant subaqueous insects, such as mayflies and chironomids, are sparse (Swammerdam, Reference Swammerdam1737; Réaumur, Reference Réaumur1742; Abel, Reference Abel1935; Scott et al., Reference Scott, Berner and Hirsch1959; Krasnenkov, Reference Krasnenkov and Hecker1966; Seilacher, Reference Seilacher1967; Illies, Reference Illies, Helmcke, Starck and Wermuth1968; Chamberlain, Reference Chamberlain and Frey1975; Kureck, Reference Kureck1996; Charbonneau and Hare, Reference Charbonneau and Hare1998; De, Reference De2002; Savrda, Reference Savrda2019), probably, in part, due to their disappearance in many modern ecosystems because of sensitivity to water pollution (Uchman et al., Reference Uchman, Mikuláš and Stachacz2017). This study finds the features of G. gingrasi n. isp. most consistent with extant mayfly burrows and argues for a subaqueous insect tracemaker for G. gingrasi n. isp. In particular, the transversely arranged bioglyphs exhibited by G. gingrasi n. isp. are comparable to those of extant subaqueous insect burrows produced by mayfly and chironomid larvae (Fig. 8).
The dominance of transverse bioglyphs along the walls of the causative tubes has been demonstrated by extant mayfly larvae burrows (Abel, Reference Abel1935; Seilacher, Reference Seilacher1967, Reference Seilacher2007; Uchman et al., Reference Uchman, Mikuláš and Stachacz2017; Fig. 8.10, 8.11) and in extant chironomid larvae burrows (Savrda, Reference Savrda2019). This transverse bioglyph feature of U- to tongue-shaped burrows appears unique to insects and thus they are considered “fingerprints” (Seilacher, Reference Seilacher2007) or “bioprints” (Rindsberg and Kopaska-Merkel, Reference Rindsberg, Kopaska-Merkel, Buta, Rindsberg and Kopaska-Merkel2005), meaning that such ichnomorphological characters (bioglyphs) can be reliably used to identify a producer. As we have noted, Glossifungites gingrasi n. isp. (Fig. 8.4–8.9) shares similar medial and lateral bioglyph orientations with extant mayfly burrows (Fig. 8.10, 8.11). Support for a mayfly tracemaker for G. gingrasi n. isp. in the Ferron Sandstone over a chironomid can be found in the size, orientation, and proportions of the burrow. Extant chironomid burrows are much smaller (maximum of 0.7 cm wide) than the G. gingrasi n. isp. herein, typically are vertically oriented unless emplaced on a vertical bank, and one aperture is noticeably larger than the other (Savrda, Reference Savrda2019). Among the taxa of extant mayfly larvae that produce subaqueous U- to tongue-shaped burrows with their specialized mandibles and front limbs are species from the families Polymitarcyidae and Ephemeridae (Edmunds and McCafferty, Reference Edmunds and McCafferty1996; Uchman et al., Reference Uchman, Mikuláš and Stachacz2017), and it appears most likely that G. gingrasi n. isp. is associated with a tracemaker from one of these two clades. Although other mayflies, such as Euthyplociidae, Ichthybotidae, Palingeniidae, Potamanthidae and Behningiidae (Miller et al., Reference Miller, Bartlett, Sartori, Breinholt and Ogden2018), are known for burrowing, they appear to be responsible for different structural emplacement or occupy different substrates.
Body fossils of mayflies, including non-fossorial forms, date back likely to the Carboniferous (McCafferty, Reference McCafferty and Grimaldi1990) and definitively to the Permian (Sinitshenkova and Vassilenko, Reference Sinitshenkova and Vassilenko2012), with fossil-calibrated molecular phylogenies suggesting an origin as far back as the Devonian (Montagna et al., Reference Montagna, Tong, Magoga, Strada, Tintori, Ho and Lo2019). McCafferty (Reference McCafferty and Grimaldi1990) identified larval forms living in flowing water (the derived ecological condition) strata from the Early Cretaceous Santana Formation, but also pointed out a lack of evidence for mayfly burrowing behavior. Polymitarcyidae body fossils are preserved in Turonian amber in New Jersey within subtropical to tropical river paleoenvironments, but extant clades have adapted more recently to inhabit temperate climates (Sinitshenkova, Reference Sinitshenkova and Grimaldi2000). The Late Cretaceous fluvial environment from which the hypodigm of G. gingrasi n. isp. derives suggests that mayflies evolved into burrowers that exploited filter-feeding from their domiciles by, at least, the Turonian. U-shaped burrows with parallel arms (Arenicolites and spreiten-bearing Rhizocorallium) have been attributed to mayfly larvae from the Triassic (Sinitshenkova et al., Reference Sinitshenkova, Marchal-Papier, Grauvogel-Stamm and Gall2005), Cretaceous (King et al., Reference King, Botterill, Gingras and Pemberton2020a), and Miocene (Fürsich and Mayr, Reference Fürsich and Mayr1981; Lange and Suhr, Reference Lange and Suhr1996; Bolliger, Reference Bolliger1999), but none of these bears evidence of diagnostic bioglyphs that would distinguish them from other tracemakers. King et al. (Reference King, Botterill, Gingras and MacEachern2020b) has suggested that the orientation in these trace fossils (G. gingrasi n. isp.) from the study area (Cowboy Canyon) is a result of an environmental preference (e.g., competition for space along the sediment-water interface, ethological alignment to maximize hydrodynamic variables for food resource, or stability).
Ichnogeny
The distal widening in larger/longer specimens of Glossifungites gingrasi n. isp. is proposed to be a result of domicile enlargement associated with organism growth. Similar size-related morphological changes attributed to organism growth are noted in many trace fossils attributed to filter-feeding organisms: Rhizocorallium (Fürsich and Mayr, Reference Fürsich and Mayr1981; Seilacher, Reference Seilacher2007), Glossifungites (Uchman et al., Reference Uchman, Bubniak and Bubniak2000), and Diplocraterion (Bromley and Hanken, Reference Bromley and Hanken1991), as well as in modern organism burrows, such as those made by mayflies (Scott et al., Reference Scott, Berner and Hirsch1959). The concept of ontogenetic changes in trace morphology has been long noted in vertebrate trace fossils that are formed at least in part by molding of a body part (Lockley, Reference Lockley, Carpenter, Hirsch and Horner1994; Olsen et al., Reference Olsen, Smith and McDonald1998; Matsukawa et al., Reference Matsukawa, Lockley and Hunt1999). More recently, the term “ichnogeny” (Belaústegui et al., Reference Belaústegui, Muñiz, Mángano, Buatois, Domènech and Martinell2016b) has been used to make reference to the origin and development over time of modern burrows or trace fossils in relation with ontogeny and/or causative behavior of the tracemaker. Morphological changes, as seen in composite forms of G. gingrasi n. isp. along the same plane (Fig. 5.3), might indicate change of angle of the burrow over time in response to some environmental variable, secondary excavation of the burrow with growth, or reoccupation (smaller scale domicile excavation) at a later time by a smaller organism.
Subaqueous excavation behavior of insects versus crustaceans
The ichnotaxonomic distinction of G. gingrasi n. isp. from that of G. saxicava, coupled with knowledge that these likely represent a difference between subaqueous insect excavation versus that of crustacean burrowing, provides a means to discern paleo-salinity conditions, since insects such as mayflies have low tolerance to persistently saline environments (Chadwick et al., Reference Chadwick, Hunter, Feminella and Henry2002; Cañedo-Argüelles et al., Reference Cañedo-Argüelles, Kefford, Piscart, Prat, Schäfer and Schulz2013). Mayfly larvae are particularly vulnerable to many stresses, including changes in temperature (Haidekker and Hering, Reference Haidekker and Hering2008; Chacón et al., Reference Chacón, Segnini and Briceño2016), sedimentation (Extence et al., Reference Extence, Chadd, England, Dunbar, Wood and Taylor2013), and anthropogenic stressors (Zedková et al., Reference Zedková, Rádková, Bojková, Soldán and Zahrádková2015), and are abundant in subaqueous, well-oxygenated, low-salinity streams (Uchman et al., Reference Uchman, Mikuláš and Stachacz2017), making them good freshwater indicators. Therefore, differentiating insect-produced burrows, such as G. gingrasi n. isp., from G. saxicava allows fluvial reaches of ancient systems to be reliably identified. Distinguishing between the two Glossifungites ichnospecies will refine interpretations of the fluvial-to-marine transition within coastal plain to nearshore systems in the rock record, potentially from the Triassic (when mayflies are first thought to have started burrowing) until recent. This realization has a direct implication on how the Glossifungites Ichnofacies is viewed with similar R-strategist filter-feeding exploitation in both the marine and fluvial systems (King et al., Reference King, Botterill, Gingras and MacEachern2020b). Furthermore, the occurrence of these large tongue-shaped traces complicates the size-diversity index (sensu Hauck et al., Reference Hauck, Dashtgard, Pemberton and Gingras2009), which generally has complex forms decreasing in diameter landward up estuaries into the freshwater realm. Fluvial systems are dynamic and considerable variation in the zone of salinity intrusion is observed in some cases. However, identification of G. gingrasi n. isp. in future studies would allow researchers to tease out complex colonization patterns in nearshore settings. These include seasonality-related fluctuations between freshwater-dominated runoff base flow conditions, when the salt wedge intrudes further into low gradient systems (La Croix et al., Reference La Croix, Dashtgard, Gingras, Hauck and MacEachern2015). Alternatively, G. gingrasi n. isp. colonization surfaces might illuminate patterns associated with increased salinity up section in nearshore fluvial channels due to channel abandonment or backing up of the system associated with transgression (Corbeanu et al., Reference Corbeanu, Wizevich, Bhattacharya, Zeng, McMechan, Chidsey, Adams and Morris2004; Richards and Bhattacharya, Reference Richards and Bhattacharya2018).
Conclusions
A new ichnospecies, Glossifungites gingrasi, is named from the Ferron Sandstone in central Utah for passively filled, horizontal to oblique, unbranched, tongue-shaped trace fossils with a medial depressed area commonly exhibiting bioglyphs with a roughly axial orientation and lateral/distal areas being dominated by bioglyphs transverse to the causative tube. The bioglyph orientation easily distinguishes G. gingrasi n. isp. from G. saxicava, with the latter having bioglyphs with a longitudinal relationship to the marginal tube. The bioglyphs are similar to the transverse ornamentation observed in the modern U- to tongued shaped burrows of subaqueous insect larvae (e.g., mayflies and chironomids). The large size of these ancient burrows in the study area suggests mayflies were the most probable producer of these specimens and that they exploited domiciles for subaqueous filter-feeding at least during, and likely prior to, the Turonian. The differentiation of freshwater insect-produced burrows G. gingrasi n. isp. from G. saxicava, the latter of which is most often associated with marginal marine producers, may provide a high-resolution tool for assessing salinity conditions in channels in the rock record globally over a large temporal period.
Acknowledgments
Thanks to Bronco Utah Operations, B. Parrish, and P. Braun for their assistance in getting access to private land for specimen collection, as well as R. Gaston for help with advice on molding and casting the axiotype and L. Herzog at the North Carolina Museum of Natural Sciences for help with specimen accession. The axiotype was molded under Bureau of Land Management permit number UT15-001S. We thank G. McDonald, R. Anderson, and the staff of the Bureau of Land Management for permitting support. Thanks to M. Ranger for the use of Applecore. Thank you to reviewers C. Savrda, P. Getty, and Z. Belaústegui, as well as Associate Editor G. Mángano, whose feedback greatly improved the quality of this manuscript. This material is based upon work supported by the National Science Foundation under Grant No. FRES 1925973. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Appendix