Introduction
Subclass Camerata Wachsmuth and Springer, 1885 is a major clade of Paleozoic crinoids comprising nearly 350 genera, including some of the earliest known crinoid taxa (Guensburg and Sprinkle, Reference Guensburg and Sprinkle2003). Camerates persisted from the Early Ordovician (Tremadocian) to the late Permian (Lopingian) and were ecologically significant constituents of lower and middle Paleozoic crinoid evolutionary faunas (CEF) (Ausich and Deline, Reference Ausich and Deline2012). Recent quantitative phylogenetic analysis of Early–Middle Ordovician crinoids indicates camerates form a monophyletic group and were the earliest clade to diverge within the Crinoidea (Ausich et al., Reference Ausich, Kammer, Rhenberg and Wright2015), making the Camerata the sister group to all other crinoids. Because of their ecological significance, taxonomic diversity, and early divergence from other crinoid clades, understanding the early evolutionary relationships among camerate lineages is important for interpreting patterns of morphologic evolution at the base of the crinoid tree of life during initial diversification of the class. Phylogenetic relationships within the Camerata remain obscure, however, and the monophyly of higher camerate taxa remains untested.
Camerates are united by the presence of rigidly ankylosed thecal and tegminal plates, fixed brachials and interbrachials incorporated into the calyx, subtegminal mouth, and typically additional plates in the posterior interray (Ubaghs, Reference Ubaghs1978). The subclass has traditionally been divided into order Diplobathrida, which is characterized by two circlets of plates (basals and infrabasals) below the radial circlet, and order Monobathrida, which possesses only one circlet of plates (basals) below the radial circlet (Moore and Laudon, Reference Moore and Laudon1943a). Although the known stratigraphic range of both orders begins in the Lower Ordovician (Tremadocian), diplobathrids went extinct at the end Mississippian (Serpukhovian) whereas monobathrids persisted until the end Permian (Lopingian). Diplobathrids are the less taxonomically diverse of the two orders, comprising roughly a third of the total generic diversity of camerates. During the Ordovician, however, diplobathrids exceeded monobathrids as constituents of the early Paleozoic CEF and in terms of taxonomic diversity, with nearly twice as many genera as the Monobathrida (Ausich and Deline, Reference Ausich and Deline2012). Following the end-Ordovician extinction, monobathrids replaced diplobathrids as the dominant camerate constituents of the middle Paleozoic CEF (Eckert, Reference Eckert1988; Ausich et al., Reference Ausich, Kammer and Baumiller1994).
Although some general patterns of camerate evolutionary history have been established, the absence of a phylogeny for the Camerata has restricted investigation of both systematic and macroevolutionary questions within the clade. Many recent studies have highlighted that phylogeny provides the valuable context of shared evolutionary history (Carlson, Reference Carlson2001; Kelley et al., Reference Kelley, Fastovsky, Wilson, Laws and Raymond2013). To this end, a phylogenetic analysis was conducted that considered all currently recognized Ordovician camerate genera to explore evolutionary relationships within the Camerata, assess the monophyly of higher camerate taxa, and evaluate the congruence of the traditional classification scheme with phylogeny. This is the first quantitative phylogenetic study to focus specifically on the Ordovician Camerata. As a result, it provides insight into a significant period in camerate evolutionary history and elucidates evolutionary relationships and patterns of morphologic evolution at the base of the crinoid tree of life. In addition to informing systematic revision of the group, the recovered trees provide a phylogenetic framework for the study of macroevolutionary patterns within the subclass Camerata.
Previous work on camerate phylogeny
The relationship of camerates to other major crinoid groups has, until recently, remained uncertain. Systematists responsible for establishing camerate classification made little attempt to infer the relationship of camerates to other crinoid groups (Moore, Reference Moore1952; Moore and Laudon, Reference Moore and Laudon1943a; Ubaghs, Reference Ubaghs1978). Since then, a number of studies have considered the relationships among the major crinoid groups, including the Camerata, on the basis of either quantitative phylogenetic analyses or qualitative study of cladograms. The major hypotheses that have been proposed for camerates are summarized by Cole and Ausich (Reference Cole and Ausich2015, fig. 1) and include past suggestions that they are most closely related to disparids (Simms, Reference Simms1993) or cladids (Ausich, Reference Ausich1998a, Reference Ausich1998b) or are sister to a clade comprised of disparids and cladids (Guensburg, Reference Guensburg2012). The most recent and comprehensive quantitative analysis, which considered Early–Middle Ordovician crinoid taxa, concluded that the Camerata: (1) form a monophyletic group, and (2) were the first clade to diverge from the rest of the Crinoidea (Ausich et al., Reference Ausich, Kammer, Rhenberg and Wright2015). It should be noted that the first point, the monophyly of the Camerata, has been supported by previous phylogenetic analyses by Ausich (Reference Ausich1998a, Reference Ausich1998b) for all camerate taxa included and by Guensburg (Reference Guensburg2012) with the exception of Eknomocrinus Guensburg and Sprinkle, Reference Guensburg and Sprinkle2003.
Although the relationship of camerates to other crinoid clades has now been established, few quantitative phylogenetic analyses have been conducted at lower taxonomic levels, and as a result, evolutionary relationships at the taxonomic rank of order and below remain poorly understood. The monophyly of orders Diplobathrida and Monobathrida have been implicitly assumed yet have never been substantiated through quantitative testing. Qualitative family-level cladograms have represented the monobathrid–diplobathrid relationship as a simple basal divergence (Moore, Reference Moore1952) or left the relationship between orders largely ambiguous (Ubaghs, Reference Ubaghs1978; Simms, Reference Simms1993; Cole and Ausich, Reference Cole and Ausich2015, fig. 2). In the most comprehensive phylogeny of Ordovician camerates, which considered Arenig to Caradoc taxa, all monobathrids and most diplobathrids were left unresolved on a polytomy, and thus the monophyly of the orders could not be established (Ausich, Reference Ausich1998b).
Our poor understanding of evolutionary relationships within the Camerata, which has been repeatedly acknowledged (Ubaghs, Reference Ubaghs1978; Simms, Reference Simms1993), is reflected in the classification of subordinal taxa. For example, the diplobathrid suborder Zygodiplobathrina (Ubaghs, Reference Ubaghs1953, Reference Ubaghs1978) is thought to represent a polyphyletic grouping although its status as a clade has never been tested through phylogenetic analysis. Similarly, the phylogenetic reality of superfamilies Dimerocrinitacea and Rhodocrinitacea have been questioned (Brower, Reference Brower1973; Frest and Strimple, Reference Frest and Strimple1981), and revisions of many camerate families to better reflect phylogeny have been proposed (Brower and Veinus, Reference Brower and Veinus1974; Kolata, Reference Kolata1982; Ausich, Reference Ausich1985, Reference Ausich1986; Rhenberg et al., Reference Rhenberg, Ausich and Kammer2015). These long-suspected systematic issues highlight the need for comprehensive camerate phylogenies to inform systematic revision and amend non-monophyletic groups.
Preliminary analysis and outgroup selection
Rooting the camerate tree
Before inferring the phylogeny of Ordovician camerates, a preliminary analysis with two primary objectives was conducted. The first goal of this preliminary analysis was to identify a suitable outgroup for the camerate phylogeny. Outgroup selection is important in phylogenetic analysis for rooting the tree and determining character polarity, which in turn may affect branching order, inferred relationships, and interpretations of morphological change within the ingroup. Using distant outgroups can result in an incorrect topology that is spuriously rooted on a long branch, and thus, the outgroup(s) selected should ideally be as closely related to the ingroup as possible to break up long branches and differentiate ancestral states from derived character states (Wheeler, Reference Wheeler1990; Smith et al., Reference Smith, Lafay and Christen1992; Smith, Reference Smith1994a, Reference Smith1994b).
Rooting the crinoid tree of life has proven one of the most challenging aspects of inferring phylogeny of the clade. This is due in part to its rapid early radiation and long-uncertain evolutionary origins, both of which complicate rooting phylogenies (Shavit et al., Reference Shavit, Penny, Hendy and Holland2007; Smith and Zamora, Reference Smith and Zamora2009). The first appearance of crinoids in the fossil record (Lower Ordovician, Tremadocian) is marked by the occurrence of representatives from several major groups, including camerates, protocrinoids, cladids, and disparids. This diversity of crinoid taxa at their first appearance precludes rooting the tree with a single crinoid taxon based on stratigraphic appearance alone. Because Camerata was the earliest recognized clade to diverge from the rest of Crinoidea, it is similarly plagued by this issue of outgroup selection (Ausich et al., Reference Ausich, Kammer, Rhenberg and Wright2015).
Recent phylogenetic analyses of pelmatozoans have suggested crinoids are nested within blastozoan clades such as diploporoids, eocrinoids, and glyptocystitoids (Sumrall, Reference Sumrall2014, Reference Sumrall2015). This relationship has been further corroborated by organic molecules recovered from fossil echinoderms (O’Malley et al., Reference O’Malley, Ausich and Chin2016). Because the potential sister group to crinoids is nested within the Blastozoa, representatives from these clades have been successfully utilized as outgroups for analyses of early crinoids (Ausich, Reference Ausich1998a, Reference Ausich1998b; Ausich et al., Reference Ausich, Kammer, Rhenberg and Wright2015). For this study, however, the paucity of unequivocally homologous characters shared between blastozoan outgroups and camerate crinoids makes it difficult to determine character polarization with certainty and avoid spurious rooting of the ingroup. Consequently, it was deemed advantageous to conduct a preliminary analysis of early camerates and other representative clades to identify an outgroup taxon that is more closely related to the camerate ingroup.
Affinities of family Reteocrinidae
The second goal of the preliminary analysis was to identify the phylogenetic position of the family Reteocrinidae Wachsmuth and Springer, 1885 relative to Camerata. The genera Reteocrinus Billings, Reference Billings1859 , Gaurocrinus Miller, Reference Miller1883, Cnemecrinus Guensburg and Sprinkle, Reference Guensburg and Sprinkle2003, and Quechuacrinus Guensburg and Waisfeld, Reference Guensburg and Waisfeld2015 are currently assigned to Reteocrinidae, which has traditionally been considered a family of diplobathrid camerates. The Reteocrinidae, however, lack some of the typical camerate characters and possess others that are idiosyncratic. Morphological traits that are atypical for most Ordovician camerates but variably possessed by reteocrinids include apinnulation, irregularly plated interrays that are not ankylosed, pentameric stems, and more than two fixed primibrachials (Table 1). Similarities have been noted between Reteocrinus and both the cladid Dendrocrinus Hall, Reference Hall1852 and the disparid Iocrinus Hall, Reference Hall1866 (Moore and Laudon, Reference Moore and Laudon1943a). On the basis of these characters, it has been suggested that reteocrinids do not belong within Camerata (Kelly, Reference Kelly1986; Donovan and Cope, Reference Donovan and Cope1989), although this proposal has not been tested quantitatively. Two of the reteocrinid genera, Cnemecrinus and Quechuacrinus, are Early Ordovician in age, making them taxa of interest for the outgroup selection analysis of early camerates. Fortuitously, the preliminary phylogenetic analysis conducted for outgroup selection also offers the opportunity to address this secondary question—affinity of family Reteocrinidae—with a single analysis.
Table 1 Morphological features of genera in family Reteocrinidae. Character states of the typical camerate condition are marked with a ‘0’; character states of the ‘reteocrinid’ condition are marked with a ‘1.’ Character states are as follows: Pinnulation: 0=pinnulate, 1=apinnulate; Interray plating: 0=regular, 1=irregular; Interray suturing: 0=ankylosed, 1=flexible; Stem: 0=holomeric, 1=pentameric; Primibrachials: 0=2, 1=greater than or equal to three. Ordovician camerates sharing reteocrinid-like features are listed.

Preliminary analysis
The preliminary phylogenetic analysis included twenty-six genera sampled from early blastozoans (six genera), cladids (four genera), protocrinoids (two genera), monobathrid camerates (five genera), non-reteocrinid diplobathrid camerates (six genera), and reteocrinids (three genera; Table 2). Taxa were coded for the preliminary analysis following the same methods used for the comprehensive analysis (see the following). The characters and character states used for the preliminary analysis were identical to those used for the comprehensive analysis, with the exception of five additional characters that were coded to encompass morphological variation of the additional taxonomic groups sampled (Supplemental Data 1). Of the 117 characters coded in total, 90 proved parsimony-informative. The six blastozoan taxa were designated as the outgroups for the analysis. A parsimony analysis was conducted in PAUP* v. 4.0a147 (Swofford, Reference Swofford2003) using a heuristic search with 1,000 random addition sequence replicates with tree bisection reconnection (TBR), holding 10 trees at each step and collapsing all branches with a maximum branch length of zero. Values for the consistency index (CI) and retention index (RI) were recorded for the recovered trees, and bootstrap values and Bremer support were calculated using PAUP*.
Table 2 Genera, species, and coding references used in the preliminary phylogenetic analysis. Asterisk (*) denotes non–type species coded due to poor preservation of the type. First appearance is given for genus.

Results and discussion
Twelve most parsimonious trees were recovered from the phylogenetic analysis with a tree length of 360 steps each and CI and RI values of 0.528 and 0.592, respectively. The strict consensus of these trees is well resolved with only small local polytomies (Fig. 1). Three monophyletic groups form that are respectively comprised of the four cladid genera, the two protocrinoid genera, and all genera traditionally considered camerates, including Cnemecrinus, Eknomocrinus, Reteocrinus, Quechuacrinus, and all other included monobathrids and diplobathrids. Although the order of branching relationships of these clades differs somewhat from the topology of Ausich et al. (Reference Ausich, Kammer, Rhenberg and Wright2015), the tree recovers the fundamental basal split between camerate and non-camerate crinoids. Within the camerate clade, Eknomocrinus and Cnemecrinus are the first to diverge, forming a clade that is well-supported by bootstrap and Bremer support values. Two reteocrinids, Reteocrinus and Quechuacrinus, form another well-supported clade. The remaining camerate taxa are not clearly divided into monobathrid and diplobathrid groups. Based on the low taxonomic sampling of camerates in this analysis and low support values for most nodes within the camerate clade, interpretations of relationships among Ordovician camerates should be made based on the subsequent analysis (see below) rather than on this preliminary analysis.

Figure 1 Strict consensus of 12 most parsimonious trees recovered from the preliminary phylogenetic analysis. Support for nodes is given by bootstrap values (above) and Bremer support (below). Labels at nodes are given for major clades; (R)=taxa traditionally placed in family Reteocrinidae.
Results indicate: (1) members of family Reteocrinidae are more closely related to camerate crinoids than to protocrinoids or cladids and thus should maintain status as camerate taxa, and (2) Eknomocrinus and Cnemecrinus form the sister group to the rest of Camerata and thus are the most suitable taxa for rooting the camerate tree.
Materials and methods
Taxon sampling and data collection
The comprehensive analysis considered all currently named Ordovician camerate genera, which includes 38 diplobathrids and 17 monobathrids (Table 3). At the outset of the study, the monobathrids Schizocrinus Hall, Reference Hall1847 and Habrotecrinus Guensburg and Sprinkle, Reference Guensburg and Sprinkle2003 were excluded due to poor preservation (<50% of characters preserved), and the incertae sedis monobathrid (?) Delgadocrinus Ausich, Sá, and Gutiérrez-Marco, Reference Ausich, Sá and Gutiérrez-Marco2007 was excluded due to its uncertain assignment to the Camerata. Thus, 52 genera in total, 38 diplobathrids and 14 monobathrids, were included in the analysis. A representative species was selected for each genus and coded using museum specimens available at the Smithsonian National Museum of Natural History, the London Natural History Museum, the Chicago Field Museum, and the Birmingham Lapworth Museum. Coding of specimens was supplemented using primary taxonomic literature (Table 3). In instances where museum specimens were inaccessible, taxa were coded using primary taxonomic literature. For each genus, the species selected for coding was typically the type species except in cases where a non–type species preserved more characters than the type or where the type species was post-Ordovician in age. Species were coded for 112 discrete characters, 64 binary and 48 multistate, that were selected to capture morphological variation among Ordovician camerates and are inferred to represent homologous structures among sampled taxa (Foote, Reference Foote1994, Reference Foote1999; Ausich, Reference Ausich1996; Kammer et al., Reference Kammer, Sumrall, Zamora, Ausich and Deline2013; Wright, Reference Wright2015) (Supplemental Data 2). Of the characters coded, 85 proved to be parsimony-informative. The Tremadocian diplobathrid Eknomocrinus was designated as the outgroup based on the results of the preliminary analysis that identified Cnemecrinus and Eknomocrinus as sister to the Crinoidea. Although both taxa are Tremadocian in age, Eknomocrinus is from a lower stratigraphic horizon than Cnemecrinus (Guensburg and Sprinkle, Reference Guensburg and Sprinkle2003). Designation of Cnemecrinus instead of Eknomocrinus as the outgroup, however, resulted in only trivial changes in ingroup topology.
Table 3 Genera, species, and coding references used in the comprehensive phylogenetic analysis. Asterisk (*) denotes non–type species coded due to poor preservation of the type; dagger (†) denotes non–type genus coded because type is Silurian. First appearance is given for genus. Family designation is based on traditional classification.

Phylogenetic analysis
To assess evolutionary relationships among the Ordovician Camerata, a parsimony analysis was conducted in PAUP* v. 4.0a147 (Swofford, Reference Swofford2003) using a heuristic search with 1,000 random addition sequences. TBR was used for the branch-swapping algorithm with no reconnection limit, and branches with a maximum length of zero were collapsed. All characters were left unordered and equally weighted. The parsimony analysis recovered 801 most parsimonious trees with lengths of 634 steps per tree. Strict consensus of the 801 most parsimonious trees resulted in a tree topology with little resolution (Supplemental Data 3). Subsequently, the characters were reweighted using the rescaled consistency (RC) function in PAUP*. This method of reweighting adjusts the weight of characters in an attempt to minimize homoplasy in the analysis (Farris, Reference Farris1989; Smith, Reference Smith1994b). After reweighting using RC, the analysis was run for 1,000 random addition sequences using the same search parameters as the initial search, and a single most parsimonious tree was recovered (Fig. 2). For the reweighted analysis, CI and RI values were recorded for the recovered tree, and bootstrap values and Bremer support were calculated using PAUP*.

Figure 2 Single most parsimonious tree recovered from the reweighted parsimony analysis with characters weighted using rescaled consistency (RC). Support for nodes is given by bootstrap values (above) and Bremer support (below). Labels at nodes represent major clades recognized herein. Unique character combinations useful for diagnosing taxa descending from each node are as follows; characters are plesiomorphic unless designated as shared derived characters by asterisks: Node A: (1) small, irregular interray plates, (2) *interrays not ankylosed, (3) posterior interray with anitaxis; (4) posterior interray with anitaxial ridge; (5) variable location of primaxil (third–fifth primibrachial), (6) 20 free arm openings, (7) apinnulate, (8) brachials rectilinear uniserial, (9) stem pentameric; Node B (Eucamerata): (1) *primaxil located on the second primibrachial, (2) *pinnulate, (3) interray plating regular, (4) stem holomeric; Node C: (1) *radials largest plates in calyx, (2) calyx high to very high; (3) posterior interray with anitaxis plating, and (4) posterior interray with anitaxial ridge; Node D (Monobathrida): (1) *one circlet below the radials, (2) basal circlet upright, (3) *radial circlet uninterrupted or only in the CD interray; Node E: (1) *fixed brachials branch twice, (2) *20 arm openings, (3) *two secundibrachials; Node F: (1) fixed brachials branch once, (2) 10 arm openings, (3) ≥ three secundibrachials; Node G (Diplobathrida): (1) two circlets below the radials, (2) concave calyx base, (3) basals partially visible in side view, (4) radials interrupted in all interrays; Node H: (1) *15–20 arm openings, (2) *two bifurcations maximum in brachials. Plate diagrams (right) correspond to representative taxa included in the analysis; black=radials; light shading=interrays; dark shading=posterior interray; cross-hatching=plating unknown (redrawn from Kolata [Reference Kolata1982; Anthracocrinus, Paradiabolocrinus], Moore and Laudon [Reference Moore and Laudon1943a; Ptychocrinus], Ubaghs [Reference Ubaghs1978; Archaeocrinus, Glyptocrinus, Reteocrinus], and Guensburg and Sprinkle [Reference Guensburg and Sprinkle2003; Eknomocrinus]).
Additional analyses
Additional character analyses were conducted in Mesquite (Maddison and Maddison, Reference Maddison and Maddison2015) for the single most parsimonious tree recovered from the reweighted analysis (Fig. 2). Using the R package STRAP (Bell and Lloyd, Reference Bell and Lloyd2015), the most parsimonious tree was plotted against the observed stratigraphic ranges of sampled genera to produce a time-scaled phylogeny (Fig. 3). Stratigraphic ranges of genera used to time-scale the tree were tabulated from Webster (Reference Webster2003) and updated to include new taxa and match current divisions of the International Chronostratigraphic Chart.

Figure 3 Time-scaled phylogeny from the single most parsimonious tree resulting from the comprehensive phylogenetic analysis. Genera with ranges extending beyond the Silurian (Neoarchaeocrinus, Givetian; Macrostylocrinus, Lochkovian) have been truncated.
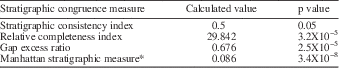
Stratigraphic congruence metrics were also calculated for the tree using STRAP to assess the fit of the recovered tree to observed stratigraphic ranges. The stratigraphic congruence measures used were the stratigraphic consistency index (SCI; Huelsenbeck, Reference Huelsenbeck1994), relative completeness index (RCI; Benton and Storrs, Reference Benton and Storrs1994), the gap excess ratio (GER; Willis, Reference Willis1999), and Manhattan stratigraphic measure* (MSM*; Siddall, Reference Siddall1998; Pol and Norell, Reference Pol and Norell2001). For each measure, the calculated value was compared to a null model of 1,000 trees generated through Monte Carlo simulation (Fig. 4), and a p-value was calculated for each of the observed metrics (Table 4).

Figure 4 Stratigraphic congruence metrics for the recovered single most parsimonious tree. For each measure, the observed value (dashed line) is compared to a null distribution produced using a Monte Carlo simulation of 1,000 trees.
Table 4 Stratigraphic congruence metrics for the single most parsimonious tree resulting from the reweighted phylogenetic analysis.
Finally, to assess the relative completeness of taxon sampling in this analysis relative to the crinoid fossil record, preservation probability and sampling rate were calculated for Ordovician camerates using methods outlined by Foote (Reference Foote1997). This approach uses the distribution of taxonomic ranges to infer maximum likelihood estimates of per-interval sampling probability, thereby minimizing potential bias of finite sample size (Foote and Raup, Reference Foote and Raup1996). All Ordovician camerate genera except Schizocrinus, Delgadocrinus, and Habrotecrinus were included in this analysis. Discrete time intervals were used based on stratigraphic ranges of genera tabulated from Webster (Reference Webster2003) updated to match current divisions of the International Chronostratigraphic Chart.
Results
Phylogenetic and other analyses
The initial equally weighted parsimony analysis recovered 801 most parsimonious trees of 634 steps in length. The topology resulting from the strict consensus of these trees was largely unresolved with only two clades of two taxa (Anthracocrinus, Strimple and Watkins, Reference Strimple and Watkins1955 and Gustabilicrinus Guensburg, Reference Guensburg1984; Ptychocrinus Wachsmuth and Springer, 1885 and Euptychocrinus Brower, Reference Brower1994) and one clade of three taxa (Atactocrinus Weller, Reference Weller1916 , Maquoketocrinus Slocom, Reference Slocom1924, and Ortsaecrinus Gil et al., Reference Gil, Domínguez, Torres and Jiménez1999) resolved (Supplemental Data 3).
The subsequent reweighted analysis, which used RC to reweight characters based on the trees recovered in the initial analysis, produced a single most parsimonious tree (Fig. 2) with a length of 70.152 and CI and RI values of 0.319 and 0.507, respectively. The four stratigraphic congruence metrics calculated for the recovered tree all proved significant, indicating the fit of the recovered tree to stratigraphy based on the observed stratigraphic ranges of taxa was significantly higher than for randomly generated trees (Table 4).
The per-interval preservation probability of camerate taxa was estimated to be 0.51, and the per-lineage-million-year sampling rate was calculated to be 0.12. The estimated sampling probability of Ordovician camerates is comparable to that of previous estimates for crinoids. For example, Foote and Raup (Reference Foote and Raup1996) calculated a per-interval preservation probability of 0.5 (equivalent to a sampling rate of 0.1) for 395 globally distributed Ordovician–Devonian crinoid genera that included both camerates and non-camerates (see Bapst and Hopkins, Reference Bapst and Hopkins2016). These estimates suggest the record is more than 70% complete at the genus level (Foote and Raup, Reference Foote and Raup1996). Thus, the taxon sampling employed within this study is interpreted herein as a highly representative sample of the crinoid fossil record.
Tree topology and clades recognized
For the reweighted parsimony analysis, the following results are presented in terms of the clades recovered, their relative placement within the tree, and diagnostic characters shared by taxa composing those clades. The major clades recovered are designated by letters at nodes in Figure 2. For each clade, unique combinations of traits are given that are diagnostic of their constituent taxa. It should be emphasized that these character combinations are comprised of both plesiomorphic and shared derived traits but are useful for diagnosing the taxa descended from each node. Characters in these combinations that represent synapomorphies (shared derived characters) are noted.
Two major clades were recovered that broadly correspond to the orders Monobathrida and Diplobathrida, with several exceptions. All diplobathrid genera form a single clade except for Rosfacrinus Le Menn and Spjeldnaes, Reference Le Menn and Spjeldnaes1996 and the four genera assigned to family Reteocrinidae (Reteocrinus, Quechuacrinus, Gaurocrinus, and Cnemecrinus). This diplobathrid clade is represented in Figure 2 by Node G and all of its descendents. All monobathrid genera form a single clade with the exception of Adelphicrinus Guensburg and Sprinkle, Reference Guensburg and Sprinkle2003 and Eknomocrinus. This monobathrid clade is represented in Figure 2 by Node D and all of its descendents. Most of the genera that do not fall within the monobathrid or diplobathrid clades are positioned near the base of the tree as taxa that are stem to the monobathrid and diplobathrid clades. These include Cnemecrinus, Eknomocrinus, Adelphicrinus, Reteocrinus, and Quechuacrinus. With Eknomocrinus designated as the outgroup, Adelphicrinus and Cnemecrinus are, respectively, the most basal lineages. If Cnemecrinus is designated as the outgroup, a clade composed of Adelphicrinus and Eknomocrinus is most basal within the tree; topology of all other ingroup taxa is identical regardless of which of the two genera is used as the outgroup (Supplemental Data 4).
Moving tipward, the two stem taxa Reteocrinus and Quechuacrinus form a well-supported clade that is diagnosed by a number of traits, including: (1) interrays comprised of small, irregular plates, (2) interray plates not ankylosed, (3) posterior interrays with anitaxis plating, (4) posterior interrays with anitaxial ridge, (5) variable location of primaxil (3rd–5th primibrachial), (6) 20 free arm openings, (7) apinnulation, (8) rectilinear uniserial brachials, and (9) pentameric stems (Fig. 2, Node A). Trait (2) is a synapomorphy, whereas all others represent plesiomorphic traits. Rosfacrinus is basally positioned relative to the division of the monobathrid and diplobathrid clades. The node that includes Rosfacrinus, the monobathrid clade, and the diplobathrid clade is well-supported by both bootstrap values and Bremer support, and taxa in this clade share the following diagnostic traits: (1) primaxil located on the 2nd primibrachial, (2) pinnulation, (3) typically regular interray plating, and (4) non-pentameric stems (Fig. 2, Node B). Characters (1) and (2) are synapomorphies, whereas (3) and (4) are plesiomorphic traits.
The monobathrid clade as recognized herein comprises 12 Ordovician taxa. The dicyclic genus Gaurocrinus plots as sister to Monobathrida and shares several diagnostic traits with the clade, including: (1) radials are largest plates in calyx, (2) calyx high to very high, (3) anitaxis plating in the posterior interray, and (4) anitaxial ridge in the posterior interray. Traits (1) and (4) are synapomorphies (Fig. 2, Node C). Diagnostic traits shared by the monobathrid clade are: (1) one circlet of plates below the radial circlet, (2) basal circlet upright, and (3) radial circlet uninterrupted or only interrupted in the posterior interray. Traits (1) and (3) are synapomorphies (Fig. 2, Node D). Pycnocrinus Miller, Reference Miller1883 is the first genus to diverge within the monobathrid clade, and the remainder of the tree is balanced in topology with two clades of five and six taxa, respectively. The characters supporting these clades are predominantly related to branching patterns in fixed brachials. The five-genus clade is united by the presence of the following: (1) fixed brachials that branch twice within the calyx, (2) 20 free arm openings, and (3) two secundibrachials, all of which represent synapomorphies (Fig. 2, Node E). The six-genus clade is united by the presence of: (1) fixed brachials that branch once within the calyx, (2) 10 free arm openings, and (3) three or more secundibrachials (Fig. 2, Node F).
The diplobathrid clade as recognized herein comprises 33 Ordovician taxa. Most morphological characters are variable across the clade, exhibiting a graded transition from states that are more similar to those of monobathrids and stem taxa. The only trait shared by all genera in the diplobathrid clade is the presence of an infrabasal circlet (Fig. 2, Node G). In addition to: (1) the presence of infrabasals, several characters are present in most genera with only a few exceptions, including (2) concave calyx base, (3) basals only partially visible in side view, and (4) interruption of the radial circlet in all interrays. Several distinct subclades are present within the diplobathrid clade, although most have poor correspondence to currently recognized families. One of the largest clades, which contains 12 genera, broadly corresponds to the family Anthracocrinidae, although some genera included in this group are currently classified as belonging to the Rhodocrinitidae. The characters uniting this clade are: (1) the presence of 15–20 arm openings, and (2) a maximum of two bifurcations in the fixed and free brachials, both of which are synapomorphies (Fig. 2, Node H). Other clades have little correspondence to traditional family divisions, being comprised of genera from the Rhodocrinitidae, Cleiocrinidae, and Dimerocrinitidae; Anthracocrinidae and Rhodocrinitidae; or strictly Rhodocrinitidae.
Discussion
Aims of the study
The objective of this study was to infer the phylogeny of the Ordovician Camerata and to use the resulting phylogeny to identify monophyletic groups, relative branching order, and patterns of morphologic evolution in early camerate crinoids. The recovered trees provide a detailed view of early evolutionary relationships among camerate crinoids and serve as a phylogenetic framework for subsequent systematic work. The recovered phylogeny of Ordovician camerates indicates systematic revision is needed at the suprageneric level. Because this study focused on Ordovician camerates, it is best suited for assessing questions related to evolutionary relationships at the base of the camerate tree of life, such as the affinity of Reteocrinidae with Camerata and the monophyly of orders Monobathrida and Diplobathrida. For this reason, the primary emphasis is on systematic revisions at the suprageneric level. Ongoing analyses of post-Ordovician Diplobathrida and Monobathrida (Cole, Reference Cole2015) are expected to further inform evolutionary relationships within these clades, and thus a comprehensive systematic revision will be postponed until more inclusive phylogenetic analyses are completed for the Camerata.
Implications for systematic revision of the Camerata
On the basis of both the preliminary analysis presented herein and the analysis of Ausich et al. (Reference Ausich, Kammer, Rhenberg and Wright2015), Camerata is recognized as a monophyletic group. As such, it should be maintained as a subclass and is formally defined by Wright et al. (Reference Wright, Ausich, Cole, Rhenberg and Peter2017). In the phylogeny of the Ordovician Camerata presented herein, the majority of taxa fall within one of two clades. These clades correspond closely to Monobathrida and Diplobathrida as historically defined, and thus, these orders should be retained based on the results of this analysis. Minor revisions, primarily reassignment of genera from each order to other groups, are required to redefine both orders as monophyletic. Formal phylogeny-based definitions are provided for Monobathrida and Diplobathrida by Wright et al. (Reference Wright, Ausich, Cole, Rhenberg and Peter2017). The terms ‘Monobathrida’ and ‘Diplobathrida’ will be used for the remainder of this discussion to refer only to taxa that belong to these clades, as identified based on the phylogenetic inferences presented herein. The remaining taxa that are not contained within Monobathrida and Diplobathrida occupy a basal position in the tree with respect to these two clades and are therefore identified as ‘stem taxa.’ Because members of the monobathrid and diplobathrid clades are more closely related to each other than to the stem taxa, it is fitting that a new term be erected to reflect evolutionary relationships between camerates and to maintain clarity in taxonomic communication. The term ‘Eucamerata’ is proposed to designate the clade containing all members of Monobathrida and Diplobathrida; stem taxa are excluded from this group. In Figure 2, Eucamerata is represented by Node B and all of its descendents. Formal definition of ‘Eucamerata’ is given by Wright et al. (Reference Wright, Ausich, Cole, Rhenberg and Peter2017). Consequently, the stem taxa identified herein are informally designated as stem eucamerates. The term ‘stem’ is here used to indicate a paraphyletic assemblage of early diverging camerate lineages with the Eucamerata as their nearest outgroup.
Taxa recognized as stem eucamerates include Eknomocrinus, Cnemecrinus, Adelphicrinus, Reteocrinus, and Quechuacrinus. Although these genera are all currently assigned to either the Monobathrida or Diplobathrida, their phylogenetic position as stem taxa indicates they should not be assigned to either of these orders. Based on the preliminary analysis, members of the Reteocrinidae are more closely related to camerates than to other crinoid groups and thus should maintain their classification as camerate taxa. Not all genera currently assigned to the Reteocrinidae form a cohesive family, however. Reteocrinus and Quechuacrinus form a clade of stem eucamerates, whereas Cnemecrinus is an independent stem lineage, and Gaurocrinus occupies a position within Eucamerata. Retention of Reteocrinus and Quechuacrinus as reteocrinids and removal of the other genera from this family would be the best approach to amend Reteocrinidae to represent a monophyletic group. The stem eucamerates form an evolutionary grade, and as such, a suite of diagnostic traits is difficult to assemble that applies to all stem eucamerates. There are several traits, however, that are commonly shared by stem eucamerates, the most notable of which include variable location of the primaxil, apinnulation, rectilinear uniserial brachials, and pentameric stems. All these traits differ between eucamerates and stem eucamerates.
As traditionally defined, most previously recognized Ordovician diplobathrid families are not supported by the tree produced in this study. The family Rhodocrinitidae, a heterogeneous group to which more than 40% of all diplobathrid genera are assigned, appears to represent a morphological grade rather than a clade. In part, this is likely because the characters used to diagnose rhodocrinitids are not unique to this family (Kolata, Reference Kolata1982; Ausich, Reference Ausich1986). One morphological character that is widely employed as a distinguishing feature of the Rhodocrinitidae is the separation of the radials in all interrays. However, several other diplobathrid families possess this character, including the Anthracocrinidae and Anthemocrinidae, both of which first appeared during the Ordovician. In this study, the largest clade within Diplobathrida comprises anthracocrinids in addition to rhodocrinitids, and most other clades include a combination of rhodocrinitid genera and genera from other families. This confirms previous suggestions that systematic revisions at the family level (Brower and Veinus, Reference Brower and Veinus1974; Kolata, Reference Kolata1982), and of the Rhodocrinitidae in particular (Ausich, Reference Ausich1986), are in order. It should be noted that several diplobathrid families, such as Dimerocrinitidae and Anthemocrinidae, have low generic diversity in the Ordovician, and thus their placement within this analysis should presently be treated with caution pending analyses that include a broader sample of post-Ordovician taxa. Similarly, the division of suborders Eudiplobathrina and Zygodiplobathrina and the superfamilies Rhodocrinitacea and Dimerocrinitacea requires sampling of post-Ordovician taxa to adequately test the validity of these groups.
As traditionally defined, the 14 Ordovician monobathrid genera included in this study are assigned to nine different families. Most of these families are represented by only a single Ordovician genus, and thus the present analysis does little to resolve the details of family-level classification. However, some family-level divisions are represented by closely related taxa, including Periglyptocrinus Wachsmuth and Springer, Reference Wachsmuth and Springer1897 and Glyptocrinus Hall, Reference Hall1847 (Glyptocrinidae); Canistrocrinus Wachsmuth and Springer, 1885 and Xenocrinus Miller, Reference Miller1881 (Tanaocrinidae); and Eopatelliocrinus Brower, Reference Brower1973 and Macrostylocrinus Hall, Reference Hall1852 (Patelliocrinidae). Likewise, the suborder Compsocrinina is represented by only two Ordovician genera, preventing confident assessment of the division between the monobathrid suborders Compsocrinina and Glyptocrinina. Ordovician monobathrids are less diverse than diplobathrids and did not become significant faunal constituents until their radiation after the end-Ordovician extinction. For this reason, the present analysis primarily aimed to test the monophyly of the Diplobathrid and Monobathrida and serves only as a preliminary analysis of other relationships among suprageneric taxa (e.g., families, superfamilies). Phylogenetic analyses including post-Ordovician taxa will be more suited to assessing the monophyly of and relationships among traditionally recognized families, superfamilies, and suborders (Cole 2017).
Patterns of early camerate morphological evolution
The inferred phylogeny of Ordovician camerates provides a framework for identifying patterns of morphological evolution within the clade. One of the most notable of these morphological characters is the monocyclic versus dicyclic condition, which has traditionally been foundational to partitioning taxa into the order Monobathrida versus the order Diplobathrida (Moore and Laudon, Reference Moore and Laudon1943a). Ancestral state reconstruction using the tree recovered from the preliminary analysis indicates dicycly is the plesiomorphic condition. This is consistent with the interpretation of Guensburg and Sprinkle (Reference Guensburg and Sprinkle2003). Order of stratigraphic appearance cannot be used to establish the ancestral condition in camerates because the first appearances of monobathrids and diplobathrids are essentially the same stratigraphically. The recovered tree indicates transitions between the monocyclic/dicyclic conditions must have occurred at least twice early in the history of the Camerata, regardless of whether Eknomocrinus or Cnemecrinus is designated as the outgroup. Among Ordovician camerates, the number of circlets appears to have later become fixed once the two major camerate clades were established. This suggests the underlying developmental pathways responsible for the monocyclic versus dicyclic condition may not have been canalized early in the history of the Camerata but later became fixed. Because of the affinity of the Floian genera Celtocrinus and Proexenocrinus with Monobathrida and Diplobathrida, respectively, this trait must have been fixed within these clades by at least the Floian. Although this does not rule out later transitions between monocycly and dicycly via reversals, as is known to have occurred in other crinoid lineages (Sprinkle, Reference Sprinkle1982b; Wright), it indicates that the presence or absence of the infrabasal circlet is highly conserved in at least Middle–Late Ordovician camerates.
A similar pattern is observed in stem eucamerates with regard to the number of primibrachial plates and the plating of the interrays. Both these characters undergo transitions from variable or irregular states in stem eucamerates to more consistent, invariable states in the Eucamerata. Complex and disordered plating has been suggested to be a basal trait of crinoids (Guensburg, Reference Guensburg2012) and is largely supported by the results of the phylogenetic analyses presented herein. In all Ordovician monobathrids and diplobathrids, two primibrachials are incorporated into the calyx, with the second primibrachial being axillary. In the stem eucamerates, however, the number of primibrachials is variable, with two in Cnemecrinus, two, three, or four in Reteocrinus, Adelphicrinus, and Eknomocrinus, and three or four in Quechuacrinus. This variability of primibrachials among genera, species, and individuals contrasts strikingly with other Ordovician camerates; the only exception is Wilsonicrinus, which has one primibrachial. Plating of the interrays follows a similar trend of irregular to regular plating across the tree. In stem taxa, interray plating is irregular in all genera except Cnemecrinus. Although irregular interray plating is not absent from Ordovician monobathrids and diplobathrids, it is an uncommon feature that is present only in restricted groups. Thus, it is possible that this character represents gradually increasing developmental constraint of interray plating during the early evolutionary history of the Camerata.
Conclusions
This study used a parsimony analysis to infer the phylogeny of the Ordovician Camerata. A preliminary analysis supported classification of Reteocrinidae as a camerate family and identified the Tremadocian camerate genera Cnemecrinus and Eknomocrinus as sister to the rest of the Camerata. A more comprehensive analysis inferred a phylogeny that serves as a framework for assessing the monophyly of higher taxa and identifying patterns of morphologic evolution within the clade. Based on the topology of the recovered tree, the traditional orders Monobathrida and Diplobathrida are supported, although minor revisions are required for them to represent monophyletic groups. Several genera are stem taxa that occupy a basal position in the tree; the monobathrid and diplobathrid clades are more closely related to each other than to these basal genera. The term ‘Eucamerata’ is proposed to represent the monobathrid–diplobathrid clade, and the stem taxa are subsequently designated ‘stem eucamerates.’ Preliminary conclusions can be made regarding suprageneric classification within the orders Monobathrida and Diplobathrida, such as the polyphyletic nature of the family Rhodocrinitidae, and regarding patterns of morphologic evolution, such as transitions between the monocyclic and dicyclic conditions.
Understanding the phylogenetic relationships of crinoids is of particular importance during the Ordovician because this time interval represents their earliest known diversification. Although camerates are a major lineage that diverged from other crinoids early in their evolutionary history, the evolutionary relationships and phylogenetic validity of suprageneric ranks have long remained untested. As the first quantitative analysis to infer the phylogeny of all well-preserved Ordovician camerate genera, this study contributes to our understanding of the crinoid tree of life and is the first step toward a comprehensive phylogenetic classification of camerate crinoids. In addition to establishing the monophyly of higher taxa within the Camerata, informing systematic revision of Ordovician camerates, and elucidating early patterns of morphological evolution within the clade, this phylogeny provides a framework for future studies of camerate diversity, disparity, morphology, and paleoecology within an evolutionary context.
Acknowledgments
I thank K. Hollis (National Museum of Natural History), T. Ewin (Natural History Museum, London), P. Mayer (Field Museum), and K. Riddington (Lapworth Museum) for access to and assistance with specimens in museum collections; S. Sheffield for assistance coding Eumorphocystis; and S. Edie for kindly providing lodging while I visited the Field Museum collections. This study and an early draft of the manuscript were greatly improved by innumerable discussions with W. Ausich and D. Wright. I thank T. Kammer and an anonymous reviewer for thoughtful reviews of the manuscript and S. Zamora for helpful comments. This research was supported in part by graduate research grants from the Paleontological Society, Sigma Xi, The Ohio State University Friends of Orton Hall fund, and the Palaeontological Association, in addition to the National Science Foundation Assembling the Echinoderm Tree of Life grant (W. Ausich, DEB 1036416).
Accessibility of supplemental data
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.js3ph.









