INTRODUCTION
The Anomalodesmata Dall, 1889 is the richest subclass of marine bivalves in terms of the variety of life habits expressed and the most specialized and often bizarre species. Their living representatives are typically rare, since many occur in deeper waters while others are restricted to highly specialized niches (Morton, Reference Morton, Wilbur, Trueman and Clarke1985a; Harper et al., Reference Harper, Hide, Morton, Harper, Taylor and Crame2000; Allen, Reference Allen2008; Morton et al., Reference Morton, Machado and Passos2015b). Long considered a monophyletic group, the anomalodesmatans are currently placed within the basal Imparidentia (Bieler et al., Reference Bieler, Mikkelsen, Collins, Glover, Gonzaléz, Graf, Harper, Healy, Kawauchi, Sharma, Staubach, Strong, Taylor, Temkin, Zardus, Clark, Guzmán, Mcintyre, Sharp and Giribet2014) and encompass 10 superfamilies, with eight occurring in the Recent fauna, that is, the Clavagelloidea d'Orbigny, 1844, Myochamoidea P.P. Carpenter, 1861, Pandoroidea Rafinesque, 1815, Pholadomyoidea King, 1844, Thracioidea Stoliczka, 1870, Cuspidarioidea Dall, 1970, Poromyoidea Dall, 1886, Verticordioidea Stoliczka, 1870, and two extinct groups – the Ceratomyoidea Arkell, 1844 and Edmondioidea King, 1850 (Runnegar, Reference Runnegar1974; Morton, Reference Morton1981a, Reference Morton2012; Bieler et al., Reference Bieler, Carter, Coan, Bouchet and Rocroi2010).
Among the estimated more than 800 Recent species of Anomalodesmata (Bieler & Gofas, Reference Bieler and Gofas2015), few have been studied alive and, as a consequence, most of the available information pertaining to their functional morphology and behaviour (lifestyle) is mainly based on preserved specimens highlighted mostly in the works of Morton (Reference Morton1973, Reference Morton1980, Reference Morton1982, Reference Morton1984a, Reference Mortonb, Reference Morton2002a, Reference Morton2003, Reference Morton2005, Reference Morton2006, Reference Morton2012, Reference Morton2015) and Morton et al., (Reference Morton, Machado and Passos2015b, Reference Morton, Machado and Passosc). Living individuals of only eight families (16 species) of Anomalodesmata have been studied, that is, the Cuspidariidae – Cuspidaria cuspidata (Olivi, 1792), Cuspidaria rostrata (Spengler, 1793), Cuspidaria obesa (Lóven, 1846) and Cardiomya planetica (Dall, 1908) (Yonge, Reference Yonge1928; Reid & Reid, Reference Reid and Reid1974; Reid & Crosby, Reference Reid and Crosby1980; Allen & Morgan, Reference Allen and Morgan1981); Penicillidae – Brechites (=Verpa) penis (Linnaeus, 1758), Brechites vaginiferus (Lamarck, 1818) and Foegia novaezelandiae (Bruguière, 1789) (Purchon Reference Purchon1955, Reference Purchon1960; Morton, Reference Morton2002b, Reference Morton2004); Lyonsiidae – Lyonsia californica Conrad, 1837, Entodesma navicula (A. Adams & Reeve, 1850) new comb. of E. saxicola and Mytilimeria nuttalli Conrad, 1837 (Yonge, Reference Yonge1952; Narchi, Reference Narchi1968); Myochamidae – Myadora striata (Quoy & Gaimard, 1835) (Morton, Reference Morton1977); Periplomatidae- Offadesma angasi (Crosse & P. Fischer, 1864) (Morton, Reference Morton1981a); Poromyidae – Poromya granulata (Nyst & Westendorp, 1839) (Morton, Reference Morton1981b); Pandoridae – Pandora filosa (Carpenter, 1864) (Thomas, Reference Thomas1994) and Frenamya ceylanica (G.B. Sowerby I, 1835) (Morton, Reference Morton1984c); and Thraciidae – Trigonothracia jinxingae Xu, 1980 and Thracia meridionalis E.A. Smith, 1885 (Morton, Reference Morton1995; Sartori & Domaneschi, Reference Sartori and Domaneschi2005). Among these, only the Cuspidariidae and Poromyidae have carnivorous representatives.
According to Bieler et al. (Reference Bieler, Carter, Coan, Bouchet and Rocroi2010), the carnivorous bivalves (Clade Septibranchia) are currently represented by three superfamilies: the Cuspidarioidea, Verticordioidea and Poromyoidea. Typically, but not wholly, these carnivorous bivalves are characterized by the presence of a muscular septum which functions in prey capture. Of these superfamilies, the Cuspidarioidea has the most representatives with about 300 species distributed in four families: the Cuspidariidae Dall, 1886, Halonymphidae Scarlato & Starobogatov, 1983, Protocuspidariidae Scarlato & Starobogatov, 1983 and Spheniopsidae J. Gardner, 1928 newly allocated by Morton et al. (Reference Morton, Machado and Passos2015b).
The Cuspidariidae stands out among the Anomalodesmata in comprising about 32% (~260 spp.) of all the species described (Gofas & Bouchet, Reference Gofas and Bouchet2015a, Reference Gofas and Bouchetb). Commonly occurring in deep and abyssal waters, the family is composed exclusively of carnivorous bivalves, which have wide geographic distributions and can generally be identified by the presence of a posteriorly rostrate shell (Allen, Reference Allen2008, Reference Allen2011; Mikkelsen & Bieler, Reference Mikkelsen and Bieler2008; Coan & Valentich-Scott, Reference Coan and Valentich-Scott2012). Despite showing great diversity in terms of anatomical characters, the cuspidariids generally have a muscular septum pierced by pores, sensory siphonal tentacles and a stomach of Type II (Yonge, Reference Yonge1928; Purchon, Reference Purchon1956; Allen & Morgan, Reference Allen and Morgan1981; Krylova, Reference Krylova1993; Poutiers & Bernard, Reference Poutiers, Bernard and Bouchet1995). According to Gofas & Bouchet (Reference Gofas and Bouchet2015a, Reference Gofas and Bouchetb), the Cuspidariidae is represented today by 18 living genera. Of these, 11 genera and 100 species have been recorded from the Atlantic Ocean while from Brazilian waters representatives of only five genera are known, that is, Cuspidaria Nardo, 1840, Cardiomya A. Adams, 1864, Plectodon Carpenter, 1865, Myonera Dall & Smith, 1886, and Octoporia Scarlato & Starogobatov, 1983 (Allen & Morgan, Reference Allen and Morgan1981; Rios, Reference Rios1994, Reference Rios2009; Absalão et al., Reference Absalão, Caetano and Pimenta2003; Absalão & Pimenta, Reference Absalão, Pimenta and Macaé2005; Absalão & Oliveira, Reference Absalão and Oliveira2011; Allen, Reference Allen2011). Oliveira & Absalão (Reference Oliveira and Absalão2009) also reported three species of Protocuspidaria Allen & Morgan, 1981, and included them as cuspidariids, but now this genus is considered in a separated family, Protocuspidariidae (Scarlato & Starogobatov, Reference Scarlato and Starogobatov1983; Krylova, Reference Krylova1995).
The genus Cardiomya is represented in Brazilian waters by only five species that are identified by rostrate shells with radial ribbing. The anatomy of Cardiomya was first described by Allen & Morgan (Reference Allen and Morgan1981) who examined the Atlantic species C. perrostrata (Dall, 1881), C. costellata (Deshayes, 1835), C. knudseni Allen & Morgan, Reference Allen and Morgan1981 and C. curta (Jeffreys, 1876) (= C. cadiziana Hubber, 2010) and, more recently, by Morton (Reference Morton2015) who described the anatomy of C. costellata. More than three decades after the last species of Cuspidariidae were observed alive, individuals of Cardiomya cleryana (d'Orbigny, Reference d'Orbigny and de la Sagra1842) have been collected from relatively shallow waters off south-eastern Brazil. This material has provided not only the first anatomical description of a Brazilian cuspidariid but also allowed for a detailed examination of the functional morphology and behaviour of living individuals of this species, providing new insights into the lifestyle of this carnivorous family of bivalves.
MATERIALS AND METHODS
Living individuals of C. cleryana were obtained from bottom samples collected with a rectangular dredge (40 × 80 and 90 cm bag length) in waters off south-eastern Brazil, specifically from the São Sebastião Channel, Araçá Bay (23°49′20.1″S 45°24′10.3″W), off the northern coast of São Paulo State by the BIOTA-FAPESP Program, between October 2012 and December 2014. Hundreds of intertidal and subtidal samples were obtained from between 3 to 20 m depths and sieved using a 0.5 mm mesh. From 10 of these, samples collected at depths of between 10–20 m, five empty shells and 33 living individuals of C. cleryana were obtained. Of these, the most active individuals were selected for behavioural observations. To do this, aquaria were filled to a depth of 15 cm with the ambient sediment (fine sand and gravel) from the collection station and then filled with seawater. Ten individuals were photographed and observed for about 2 h. Some were also videotaped. Behaviours, such as the digging process, digging depth and siphonal movements were recorded. Other individuals were anaesthetized with menthol and magnesium chloride and dissected to remove the mantle, foot and adductor muscles (for future molecular analysis) and male gonads (for future TEM analysis). Other individuals were selected for SEM examination of the shells and internal tissues. For histological purposes, the most relaxed individuals were decalcified in a solution of 100 ml distilled water containing 0.89 g of NaCl and 1.02 g of ascorbic acid and embedded in Historesin® in order to obtain serial transverse and sagittal sections of between 3–5 µm thick. All specimens, SEM stubs and histological slides are deposited in the Museum of Zoology ‘Prof. Adão José Cardoso’ of the University of Campinas (ZUEC), with the following accession numbers: ZUEC-BIV 5119–5141.
RESULTS
SYSTEMATICS
Order ANOMALODESMATA Dall, 1889
Superfamily CUSPIDARIOIDEA Dall, 1886
Family CUSPIDARIIDAE Dall, 1886
Genus Cardiomya A. Adams, 1864
Cardiomya cleryana (d'Orbigny, 1842)
(Figures 1–10)
Cardiomya A. Adams, 1864
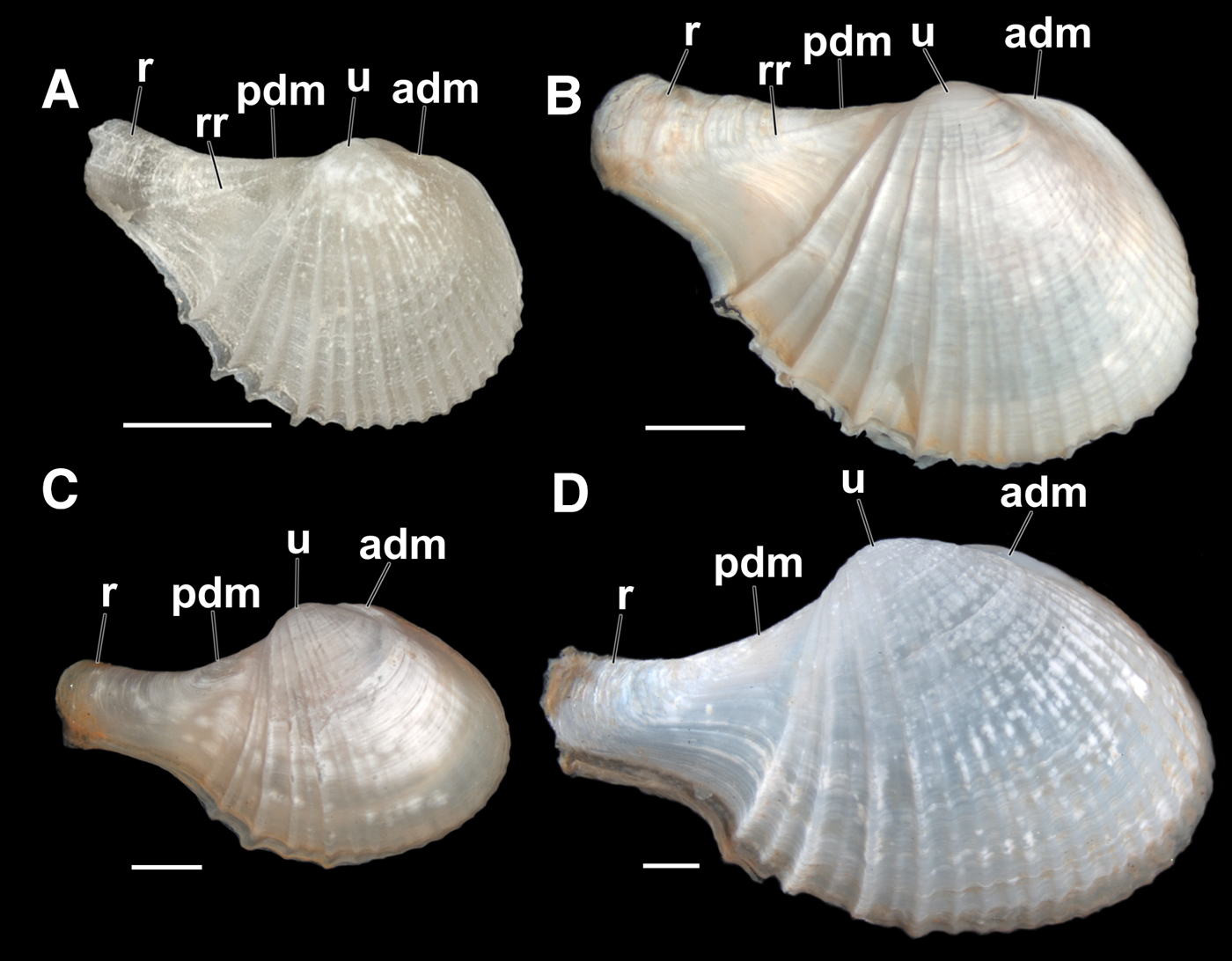
Fig. 1. Comparison between the shells of Cardiomya perrostrata and Cardiomya cleryana. (A, B) outer view of right valve of two specimens of C. perrostrata, with straight dorsal margins, umbones smaller and low with a straighter, thicker and slightly point up rostrum; (A) a specimen with ~2.4 mm in length – USNM 832408; (B) a specimen with ~7.3 mm in length – ZUEC-BIV 5130. (C, D) outer view of right valve of two specimens of C. cleryana, showing the contour of dorsal margins, umbones more prominent with rostrum thinner and slightly recurved. (C) a specimen with ~6.3 mm in length – ZUEC-BIV 5133; (D) a specimen with ~11.7 mm in length – ZUEC-BIV 2218. (See the list of abbreviations). Scale bars: A–D, 1 mm.
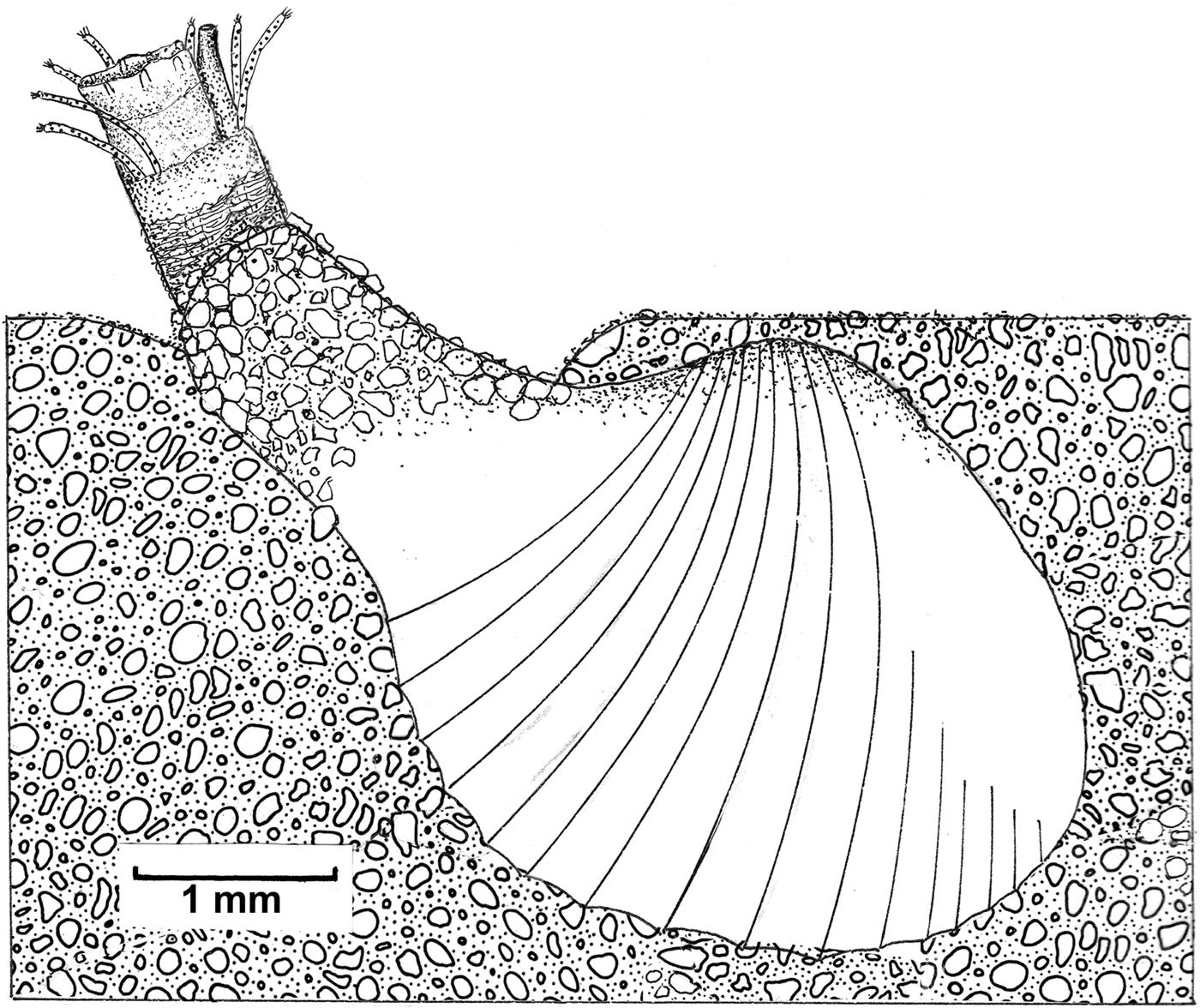
Fig. 2. Cardiomya cleryana. An adult individual in its life position in the sediment.
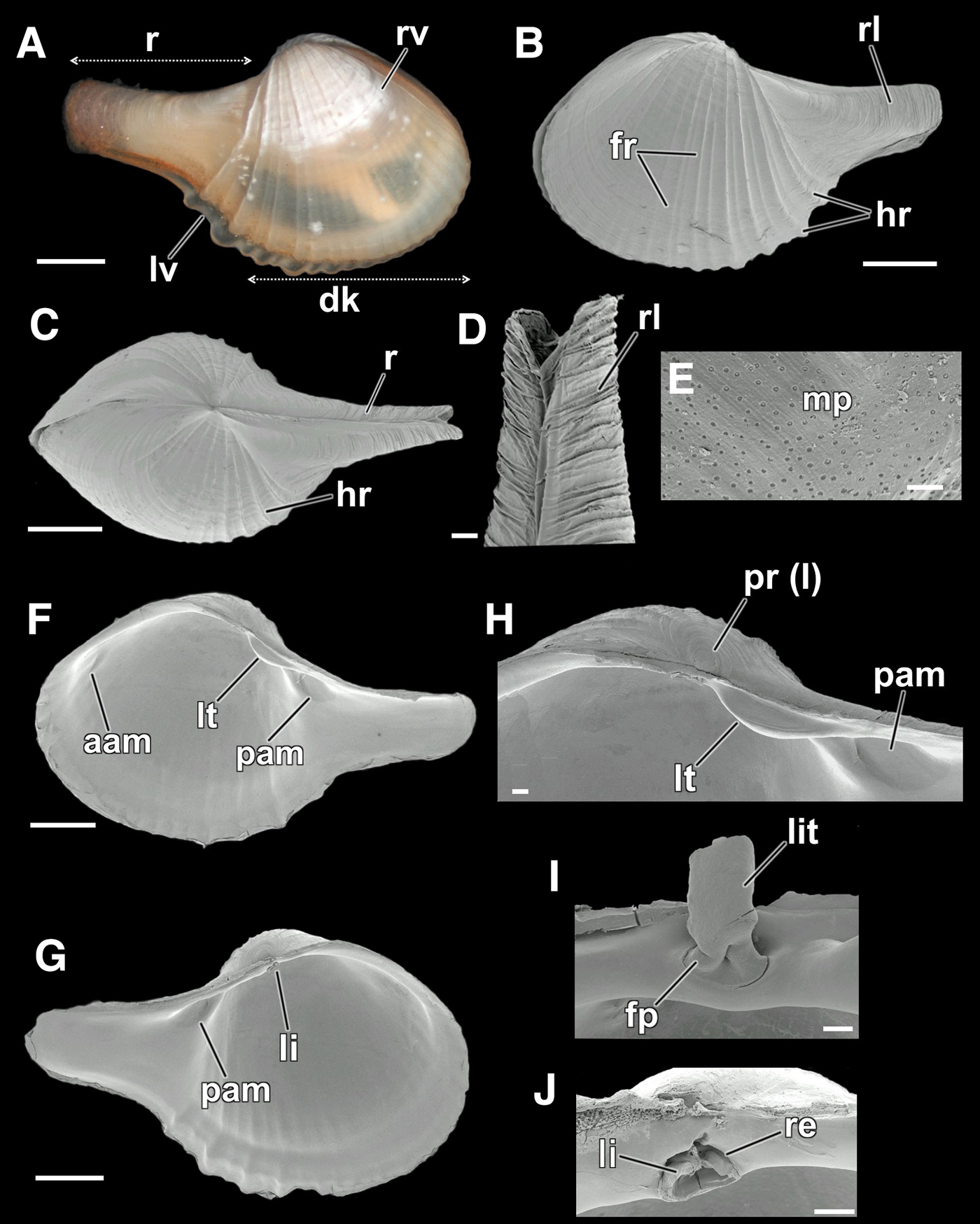
Fig. 3. Cardiomya cleryana, details of shell. (A) photomicrograph of a living specimen (right view) with the limits of disk and rostrum, showing the overlap between the valves – ZUEC-BIV 5132; (B-J) SEM views – ZUEC-BIV 5119, 5120; (B) external view of the left valve, showing the disk decorated with fine and heavy radial ribs and the rostrum with fine radial lines; (C) dorsal view; (D) higher magnification of rostrum with radial lines; (E) magnification of dissoconch, near its limits with the prodissoconch, showing micropits; (F) inner view of right valve, showing the posterior lateral tooth and the adductor muscle scars; (G) inner view of left valve, with ligament bellow the umbo; (H) magnification of the right hinge plate, showing the prodissoconch I, posterior lateral tooth and adductor muscle scar; (I) ligament, with its calcified (lithodesma) and fibrous parts; (J) resilifer with a part of the ligament. (See the list of abbreviations.) Scale bars: A–C, F, G, 1 mm; D, H, I, J, 100 µm; E, 10 µm.

Fig. 4. Cardiomya cleryana, details of anatomy. (A) photomicrograph of a living specimen with the siphons protruded; (B) magnification of siphons, showing the seven siphonal tentacles (four in the inhalant and three in the exhalant) and one set of inter-tentacular projections between the exhalant siphonal tentacles. (C–F) SEM views of siphons – ZUEC-BIV 5123, 5124. (C) an exhalant inter-tentacular projection, formed by three small papillae; (D) frontal view of the siphonal apparatus with inhalant and exhalant siphons, showing the siphonal sheath; (E) siphonal tentacle, with micro-papillae in the surface and glandular papillae in the surface of siphons; (F) apical ciliated tip, showing cilia inside. (G–H) histological sections – ZUEC-BIV 5139. (G) transverse section of siphons, showing the arenophilic glands; (H) sagittal section of arenophilic layer, showing the glandular papillae, duct and core; (I) dissected specimen – ZUEC-BIV 5127, showing the septum with four pairs of pores, mouth and septal pedal opening; (J) higher magnification of one septal pore, with internal ring of cilia. (See the list of abbreviations.) Scale bars: A, 1 mm; B, 200 µm; C, 30 µm; D, J, 100 µm; E, F, 10 µm; G, 200 µm; H, 50 µm; I, 500 µm.
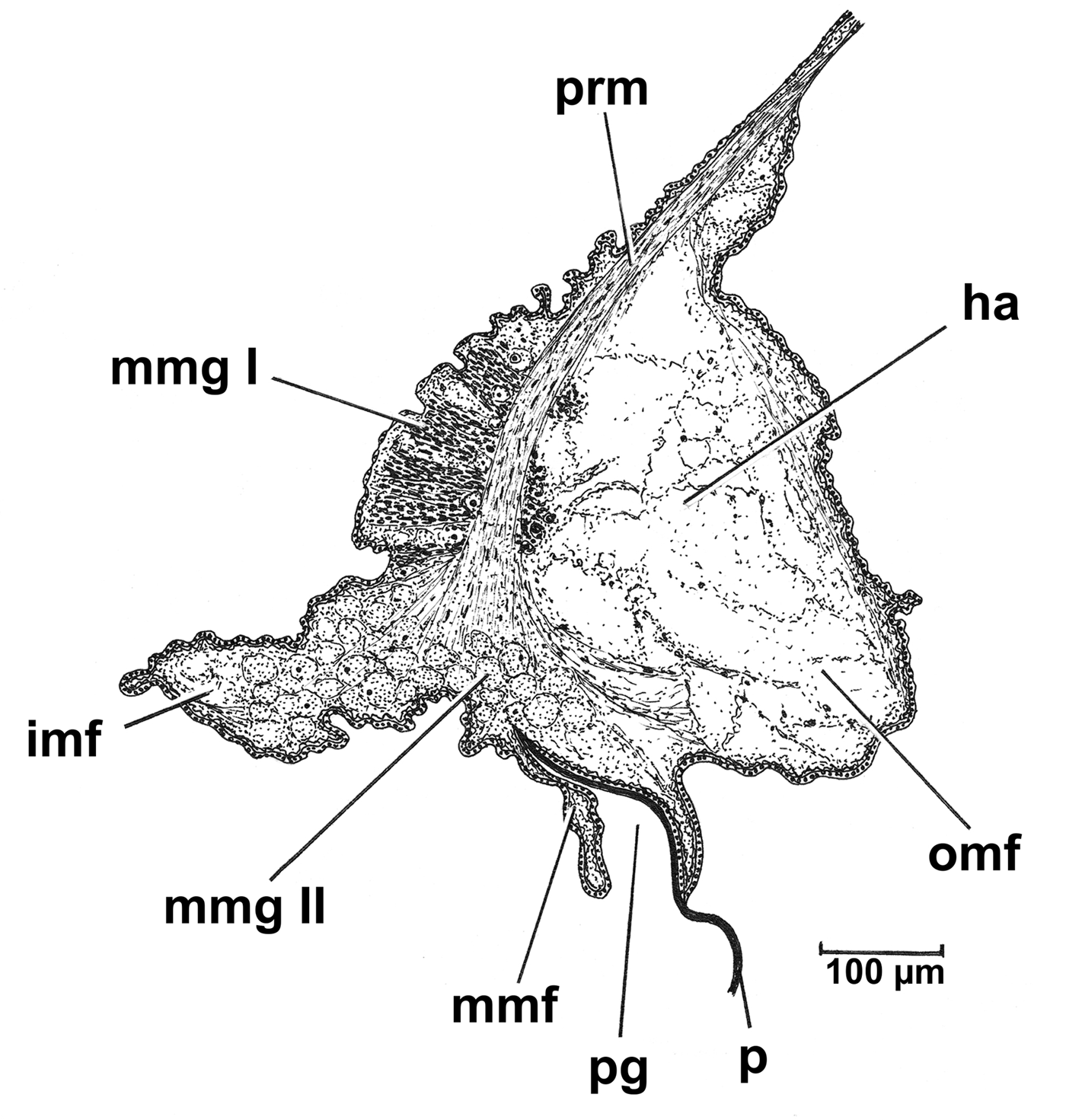
Fig. 5. Cardiomya cleryana. Transverse section through right mantle margin. (See the list of abbreviations.)

Fig. 6. Cardiomya cleryana. An adult individual as seen from the right side, with the right shell valve and mantle lobe removed to illustrate the organs of the mantle cavity and visceral mass. (See the list of abbreviations.)

Fig. 7. Cardiomya cleryana, details of anatomy. (A-B) photomicrographs of two living specimens. (A) a female, showing pink ovaries; (B) a male with whitish testes; (C) sagittal section in a single ovarian follicle, showing the follicular wall, lumen and oocytes in different stages of maturity; (D) transverse section of testes, showing spermatogonia, spermatocyte and spermatozoa; (E) living specimen with the protruded extensible foot. (F–G) SEM images – ZUEC-BIV 5127. (F) ventral view of foot showing the pedal groove; (G) higher magnification of the pedal groove, showing the cilia. (H–I) histological sections of the foot – ZUEC-BIV 5139. (H) sagittal section showing dorsally the digestive diverticulae, septum, supraseptal chamber, septum membrane, byssal gland with the two glandular regions and the formation of a single byssal thread; (I) transverse section, showing the byssal gland region I, pedal ganglia and statocysts with small, irregular and crystal-like statoconia inside. (See the list of abbreviations.) Scale bars: A, B, E, 1 mm; C, 100 µm; D, 50 µm; F, H, 200 µm; G, 20 µm; I, 50 µm.

Fig. 8. Cardiomya cleryana. An adult individual in its natural position in the sediment, with the elongated foot and the anchoring byssal thread. (See the list of abbreviations.)

Fig. 9. Cardiomya cleryana, details of anatomy. (A) sagittal section from the left side without siphons, showing details of organs of pallial cavity and visceral mass – ZUEC-BIV 5139. (B–E) photomicrographs made from dissected specimens – ZUEC-BIV 5127, 5129. (B) anteroventral view showing the mouth with labial palps, septal pores and septal pedal opening; (C) right view showing some organs of pallial cavity and visceral mass; (D) posteroventral view showing the posterior septal muscle, retractor pedal muscle bifurcated before attachment in the shell, posterior adductor muscle, connectives nerve bundles, hind gut, rectum and visceral ganglia; (E) dorsal view of the buccal funnel after removal of the anterior adductor muscle, showing the cerebropleural ganglia and its branches. (See the list of abbreviations.) Scale bars: A, E, 500 µm B, 200 µm; C, D, 1000 µm.

Fig. 10. Cardiomya cleryana. A transverse section through the pedal ganglia and paired statocysts at the base of the foot. (See the list of abbreviations.)
Cardiomya has an inflated globular to ovate shell, with an elongated rostrum. The sculpture is of strong radial ribs posteriorly and commarginal striae. Right valve with a posterior lateral tooth, which may be obsolete; left valve edentate. Resilifer shallow to deep, sub-vertical. Septum muscular with four pairs of pores and small lateral septal muscles attached close to the anterior end of the posterior siphonal retractor muscles (Poutiers & Bernard, Reference Poutiers, Bernard and Bouchet1995; Coan et al., Reference Coan, Valentich-Scott and Bernard2000; Coan & Valentich-Scott, Reference Coan and Valentich-Scott2012).
ORIGINAL DESCRIPTION
Sphène de Cléry (Homage to Mr Cléry) or Sphena cleryana d'Orbigny, 1845: p. 572, pl. LXXXIII, figures 16–18 (d'Orbigny, Reference d'Orbigny1834–Reference d'Orbigny1847) or in d'Orbigny, Reference d'Orbigny and de la Sagra1842–Reference d'Orbigny and de la Sagra1853: p. 285.
SYNONYMY
Sphena cleryana d'Orbigny, 1846; Cuspidaria simillima E.A. Smith, 1915; Cuspidaria (Cardiomya) simillima E.A. Smith, 1915.
List of abbreviations used in the figures
aam, Anterior adductor muscle (or scar); adm, Anterior dorsal margin; ag, Arenophlic gland; an, Anus; ap, Anterior labial palp; aprm, Anterior pedal retractor muscle; asm, Anterior septal muscle; bg, Byssus gland; bg I, Byssal gland type I; bg II, Byssal gland type II; bn, Buccal nerve; bt, Byssal thread; c, Core of the arenophilic gland; cg, Cerebropleural ganglia; ci, Cilia; cn, Comissure nerve; cnb, Connectives nerve bundles; css, Crystalline style sac; dd, Digestive diverticulae; dg, Duct of arenophilic gland; dk, Disk (shell); es, Exhalant siphon; et, Exhalant tentacle; emo, Early maturing oocyte; f, Foot; fl, Follicular lumen; fp, Fibrous part of ligament; fr, Fine radial ribs; f (tw), Foot twisted; fw, Follicle wall; g, Gonad; gp, Glandular papillae; gs, Gastric shield; h, Heart; ha, Haemocoel; hg, Hindgut; hr, Heavy radial ribs; imf, Inner mantle fold; ipi, Ingested prey item; io, Immature oocyte; is, Inhalant siphon; ise, Intersiphonal septum; isc, Infra-septal chamber; it, Inhalant tentacle; ip, Inter-tentacular projections; li, Ligament; lit, Lithodesma; ln, Labial nerve; lt, Lateral tooth; lv, Left valve; m, Mouth; mg, Midgut; mm, Mantle margin; mmf, Middle mantle fold; mmg I, Mantle margin gland I; mmg II, Mantle margin gland II; mmn, Muscular nerve; mo, Mature oocyte; mp, Micropits; o, Oesophagus; ov, Ovaries; omf, Outer mantle fold; p, Periostracum; pc, Pericardial cavity; pg, Periostracal groove; pp, Posterior labial palp; pam, Posterior adductor muscle (or scar); pdm, Posterior dorsal margin; peg, Pedal groove; pega, Pedal ganglia; pest, Posterior extension of stomach; pga, Pedal gape; psm, Posterior septal muscle; pr I, Prodissoconch I; prm, Pallial retractor muscle; pprm, Posterior pedal retractor muscle; pvo, Pre-vitellogenic oocyte; r, Rostrum; rc, Rectum; re, Resilifer; rl, Radial lines; rr, Rostral ridge; rv, Right valve; s, Siphons; se, Septum; sm, Septal membrane; sep, Septal pore; sep (1)–(4), Septal pores (1–4); spg, Septal pedal gape; spm, Spermatocyte; spo, Spermatogonia; spz, Spermatozoan; spi, Sphincter of inhalant siphon; ss, Siphonal sheath; ssc, Supra-septal chamber; st, Stomach; sta, Statocyst; stc, Statoconia; te, Testes; tp, Tentacle papillae; to, Tip opening in the siphonal tentacle; u, Umbones; vg, Visceral ganglia; vn, Visceral nerve; vm, Visceral mass.
TYPE LOCALITY
Cape St. Thomé Peninsula, Campos dos Goytakazes – off Rio de Janeiro State, Brazil from 80 m depth.
CONCHOLOGICAL REMARKS
According to Gofas & Bouchet (Reference Gofas and Bouchet2015a, Reference Gofas and Bouchetb), 53 recent species constitute the genus Cardiomya. Of these, the Pacific species C. lanieri (Strong & Hertlein, 1937), C. gouldiana (Hinds, 1843) and C. pectinata (P.P Carpenter, 1864) most resemble C. cleryana particularly in terms of the outline and arrangement of the radial ribs. Although similar, however, some significant conchological differences can be identified, for example, the presence of two sharp and widely spaced radial ribs in the posterior portion of the disk of C. lanieri; the presence of regular commarginal lirae between all the ribs and a reduced rostrum in C. gouldiana and the undifferentiated shape of the ribs in C. pectinata (Poutiers & Bernard, Reference Poutiers, Bernard and Bouchet1995, figures 59, 60; Coan & Valentich-Scott, Reference Coan and Valentich-Scott2012, plate 318; Coan et al., Reference Coan, Valentich-Scott and Bernard2000, plate 120).
Only seven species of Cardiomya are known to occur in the Western Atlantic Ocean. Of these, according to Rios (Reference Rios1994, Reference Rios2009) and Absalão & Pimenta (Reference Absalão, Pimenta and Macaé2005), only five species occur in Brazilian waters. These are: C. cleryana, C. ornatissima (d'Orbigny, 1853), C. perrostrata (Dall, 1881), C. striata (Jeffreys, 1876) and C. surinamensis van Regteren Altena, Reference Altena and Van1971. Among the possible Brazilian species, C. ornatissima is different from the others in having prominent radial ribs with broad interspaces, resembling C. costata (G.B. Sowerby I, 1834), which occurs from Baja California to Ecuador, and C. glypta Bush, 1898, which occurs off French Guiana. Both of these taxa are illustrated by Coan & Valentich-Scott (Reference Coan and Valentich-Scott2012, plate 317) and Massemin et al. (Reference Massemin, Lamy, Pointier and Gargominy2009, p. 349), respectively. No other Brazilian records have been identified with regard to C. striata and C. surinamensis other than Rios (Reference Rios1994, figure 1486; Reference Rios2009, figure 1681) and Absalão & Pimenta (Reference Absalão, Pimenta and Macaé2005, figure 135), respectively; and though similar to C. cleryana in the number of ribs, C. surinamensis has an oval shape and a much reduced rostrum (Altena, Reference Altena and Van1971, figure 3).
Cardiomya cleryana and C. perrostrata are the most well known taxa from Brazilian waters and are similar to each other and easily confused. After analysing photographs of museum lots (NMR – Natuurhistorisch Museum, Rotterdam, Holland; ZUEC – Museum of Zoology ‘Prof. Adão José Cardoso’ of the University of Campinas, Brazil; USNM – United States National Museum, Smithsonian Institution, USA) containing shells of different sizes of these two species this paper describes and illustrates some characteristics that help in their differentiation. It is also worth noting that there are no significant differences between their hinge plates. We, therefore, highlight four distinctive shell characters that separate them: the contours of the posterior-anterior dorsal margin, the shape of the rostrum, the presence or absence of a rostral rib and the position of the umbones. Individuals of C. perrostrata are on average smaller than congenerics (2.4–7.3 mm in shell length) and have a straight and continuous postero-dorsal shell margin (pdm), confluent with the rostrum (r); a straight antero-dorsal margin (adm) forming a small shoulder more obvious in larger individuals; a straight, thick and slightly upwardly pointing rostrum with one diagonal rostral ridge (rr) on the surface and umbones (u) small, low and positioned more medially (Figure 1A, B). Cardiomya perrostrata is, therefore, different from C. cleryana (4.5–11.8 mm in shell length), which has rounded anterior and slightly concave posterior dorsal shell margins, a thin, slightly recurved, rostrum and more prominent umbones slightly displaced towards the posterior (Figure 1C, D).
BIOLOGY AND BEHAVIOUR
Distribution
Rio de Janeiro to Tierra del Fuego and the Falklands Islands (Carcelles, Reference Carcelles1950; Rios, Reference Rios1994, Reference Rios2009; Scarabino, Reference Scarabino2003). Specifically with regard to Brazil: Bahia de Paranaguá (25°30′S 48°30′W), Paraná State (Boehls et al., Reference Boehls, Absher and Cruz-Kaled2004); Ilha Grande Bay (44°45′W 23°20′S), Angra dos Reis, Rio de Janeiro State (Grillo et al., Reference Grillo, Ventura and Silva1998); Channel of São Sebastião, Ponta do Guaecá (23°50′S 45°27′W) to Baía do Araçá (23°48′S 45°24′W), 5–10 m, São Paulo State (Migotto et al., Reference Migotto, Tiago and Magalhaens1993). This species was also originally reported upon from Jamaica, Cuba, St. Thomas, Guadalupe (d'Orbigny, Reference d'Orbigny and de la Sagra1842–Reference d'Orbigny and de la Sagra1853).
The living animal
SIPHONAL MOVEMENTS
Ten living individuals of C. cleryana were observed in relation to their siphonal movements and five of these also had these filmed with high-resolution cameras providing the first such record of a carnivorous bivalve (see video in Supplementary material). This tool was important for the interpretation of the complex movements exhibited by the siphons. The siphons of C. cleryana are sensitive to vibrations in the water column and these and other mechanical disturbances caused their rapid retraction within the rostrum. When they re-emerged, the seven sensory tentacles and the two sets of exhalant inter-tentacular projections were also extended. In living individuals it was also possible to see the colour of the siphonal sheath, which was also extended from the posterior margins of the rostrum. Even though buried at 45° to the sediment-water interface, the siphons of C. cleryana are always extended in a near-vertical position.
Three sequential siphonal movements undertaken by C. cleryana were observed: (i) water circulation, (ii) cleaning and (iii) feeding.
-
(i) The circulation of water into and out of the mantle cavity of C. cleryana was characterized by the simultaneous extrusion of the inhalant and exhalant siphons, creating currents that circulate through the infra- and supra-septal chambers via the septal pores. This movement was not constant, occurring at intervals of between 5–10 s and was synchronized with movements of the muscular septum that creates these currents. When the exhalant siphon was extended its length was greater than that of the inhalant whereas the latter had a much wider aperture. It is noteworthy that relaxation of the septum resulted in the retraction of the exhalant siphon, the two sets of inter-tentacular projections thereby closing the exhalant opening. The projections resemble two small opercula that open and close according to the status of the exhalant siphon. This movement possibly assists in the oxygenation of tissues and removes faeces and other nitrogenous wastes.
-
(ii) A cleaning action was observed during the digging process of C. cleryana and appears to be associated mainly with removing sediment and pseudofaeces accumulated in the infra-septal chamber. This movement was performed exclusively by the inhalant siphon and lasted for but 1–2 s. It resulted in the extension of the inhalant siphon while simultaneously reducing its diameter, creating a more elongate but narrower tube. The simultaneous depression of the septum increases the pressure in the infra-septal chamber creating this action and which in conjunction with valve closure results in sediment and/or pseudofaeces being ejected from the inhalant siphon thereby cleaning the infraseptal chamber. Throughout a digging cycle this movement was repeated two to three times.
-
(iii) Feeding is uniquely associated with prey capture in C. cleryana and was more difficult to observe, because it was typically confused with water circulation movements. Feeding was only observed in completely buried individuals and it was thus not possible to observe the associated movements of the muscular septum. During feeding, however, the aperture of the inhalant siphon was narrowed and pointed towards potential prey, capturing it by means of the dramatic extension and opening of the siphonal aperture, as described and illustrated for C. planetica by Reid & Crosby (Reference Reid and Crosby1980, figure 1).
THE DIGGING AND RE-BURIAL PROCESS
Cardiomya cleryana naturally occurs in a sediment characterized by a mix of fine sand and gravel. A change in the type of sediment appears to affect the ability to dig in this species. After being collected, 10 individuals of C. cleryana were kept in aquaria without sediment but with running seawater for 30 min. The most active individuals of these were then placed in Petri dishes with a thick bed of sediment. Some individuals were filmed. During these observations, three combinations of sediment were offered, that is, fine sand + gravel (original combination), gravel only and fine sand only. When exposed to the original sediment (fine sand + gravel) most individuals buried themselves within 6–7 min. In gravel, no individuals buried themselves whereas in fine sand most re-buried within 2–4 min. No significant differences were observed either between adults and young individuals or between males and females. All the observed individuals behaved in the same way during the re-burial process. The digging sequence can be divided into three parts: (i) recognition of sediment type, (ii) pedal digging movements, and (iii) final positioning in the sediment as follows (see video in Supplementary material).
-
(i) When placed on the sediment, the foot of each individual was extended from the pedal gape to touch the sediment and was then retracted immediately into the infra-septal chamber bringing with it some of the particles. Possibly, this helps the bivalve to evaluate grain size and thus its resistance to re-burial. The same movement could be repeated several (2–20) times and may or may not result in re-burial. When exposed to fine sand, the individual's foot made similar movements but digging movements commenced immediately.
-
(ii) When digging commenced, the foot penetrated the sediment and initially turned an individual onto its side. As the foot began the re-burial process, contraction of the pedal retractor muscles straightened the shell so that the ventral margin was in contact with the sediment surface. From this point onwards, the foot began to burrow deeper and deeper and the pedal retractor muscles interacted with movements of the septum to complete the re-burial process in a series of downward oriented jerky movements. These intermittent movements were not continuous but occurred at intervals of about 2–3 s. The direction of the excavation was always the same for all individuals, that is, the anterior region was buried first and, then, in a series of antero-posterior rocking movements, the shell was eventually positioned at 45° to the sediment surface. Cardiomya cleryana continued the re-burial process until only ~1/3 of the rostrum was exposed above the sediment surface. This region of the shell and siphonal sheath is camouflaged within the sediment by the adhesion of sand grains to it using arenophilic gland secretions.
-
(iii) After removing each of the individuals carefully from the sediment, it was clear that they were all buried at an angle of ~45° (Figure 2), different to the vertical position (90°) observed by Yonge (Reference Yonge1928) for Cuspidaria obesa and Cuspidaria cuspidata and for Cuspidaria rostrata by Reid & Reid (Reference Reid and Reid1974) and as generally postulated by Morton (Reference Morton1987, figure 13) for species of Cardiomya and Cuspidaria. In addition to observing position in the sediment, burial depth was measured and this was shown to approximate individual shell length.
ANATOMY
The shell
Shell small (length: 4.5–11.7 mm; height: 3–6 mm; width: 2.3–5.1 mm), elongate, pear-shaped, inequilateral, slightly inequivalve, rostrate, inflated (Figure 3A–C); shell valves thin, whitish to somewhat transparent; left valve (lv) larger than the right (rv) and overlapping it ventrally (Figure 3A). Rostrum (r) moderately narrow (~800–1100 µm), long (~1/2 the length of the shell), slightly recurved, subtruncate (Figure 3A–C); with sinuous radial lines (rl) on the external surface (Figure 3B, D). Umbones prominent and slightly displaced to the posterior. Prodissoconch I (pr I) small (~130 µm in length), circular and smooth; limits of prodissoconch II not visible (Figure 3H). Antero-dorsal shell margin small, rounded, merging with the umbones and confluent with the anterior margin; posterodorsal shell margin larger than the anterior, straight to slightly concave, confluent with the posterior margin and merging with the rostrum (Figure 3F, G). In some individuals the anterior dorsal margin may be slightly prominent and convex (Figure 3B, F, G). Lunule and escutcheon absent. Periostracum thin, adherent and translucent white to light brown (Figure 3A). External surface of the valves with fine commarginal striae, stronger ventrally; main body of the shell or disk (dk) characterized by 13–20 complete radial ribs which extend from close to the umbones of each valve to the ventral margin; anterior slope with 10–15 fine and narrow radial ribs (fr); posterior portion with 3–5 heavy radial ribs (hr) with short interspaces, sometimes with fine radial ribs (Figure 3A–C). Micropits (mp) present but restricted to around prodissoconch I, only visible with SEM (Figure 3E). Inner surface whitish and smooth, anterior slope and ventral margin with crenulations corresponding in position with the external ribs. Hinge plate very narrow. Right valve with only one strong, moderately short, posterior lateral tooth (lt) (Figure 3F, H); left valve edentulous (Figure 3G). Amphidetic ligament (li) fibrous (fp) and with a calcified lithodesma (lit), deeply located on a subtriangular resilifer (re) placed directly below the umbones (Figure 3I, J). Anterior adductor muscle scar (aam) elongate, barely visible; posterior adductor muscle scar (ppm) more rounded than the anterior, deep and well marked (Figure 3F–H). Pallial line entire and not visible, even with SEM.
The siphons
Posteriorly, the siphonal apparatus (sa) comprises two siphons, inhalant (is) and exhalant (es), surrounded by small sensory tentacles and encased in a thick and muscular siphonal sheath (ss) (Figure 4A, B & D). The siphons are formed by fusion of the inner folds and inner surfaces of the middle mantle folds (type B of Yonge, Reference Yonge1957) and are separate. This separation occurs by means of a specific structure, the inter-siphonal septum, a delicate posterior extension of the muscular septum. This same extension also forms the sphincter at the base of the inhalant siphon, which is dorsally attached to the posterior septal retractor muscle. The inhalant sphincter is important in regulating the water flow and input of prey into the infra-septal chamber. In anaesthetized individuals, the sphincter measures ~570 µm in length and 340 µm in diameter.
The siphons are supported by a set of muscle bundles, peripherally traversing the entire length of the siphonal apparatus and terminating near the posterior septal muscle as the siphonal retractor muscles. The inhalant siphon is large and possesses four finger-shaped sensory tentacles (it) arising from its ventral and lateral base. The exhalant siphon is ~1/3 smaller in diameter than the inhalant, with three tentacles arising from its latero-dorsal base. Also identified are two projections attached to the free edge between the exhalant siphonal tentacles (et). These inter-tentacular projections (ip) are formed by three small papillae resembling siphonal tentacles still in the process of formation (Figure 4B–D). Similar structures have been observed previously in some species of Bathyneaera (Cuspidariidae) (Krylova, Reference Krylova1993).
Using SEM, the opening at the tip (to) of each siphonal tentacle comprises a pit containing a bundle of cilia (ci) (Figure 4F). The seven siphonal tentacles are similar in shape and size and measure about 100 µm in length. In living individuals, the tentacles are pigmented with small white spots. Using SEM, it is possible to observe the presence of micro-papillae (tp) on the external surfaces of the siphonal tentacles, these being larger and more prominent on the lateral and ventral faces (Figure 4E). Such micro-papillae were not observed on the surfaces of the inter-tentacular projections.
In cross-section, the siphons are formed by five tissue layers: (i) an outer epithelium characterized by the presence of cylindrical cells; (ii) an arenophilic layer with numerous arenophilic glands; (iii) a layer of circular muscles; (iv) an innermost layer of longitudinal muscles overlying; and (v) the internal squamous epithelium. The arenophilic glands (ag) are characterized by a core (cg) and a duct (gd) that in living individuals extends outwards forming a small U-shaped papilla (Figure 4G, H). In fixed individuals these glandular papillae and their ducts are inverted (Oliveira & Sartori, Reference Oliveira and Sartori2013, figure 3). Using SEM, the glandular papillae can be observed easily on the surface of the siphons and are cylindrical in shape sometimes with a constriction apically (Figure 4E).
The siphonal sheath that surrounds the siphons is a tubular extension of the outer fold of left and right mantle lobes that extend from the posterior end of the septum into the rostrum. In living individuals, the most posterior portion of the sheath is pigmented with small white spots as is the inhalant siphon and its tentacles. Anteriorly, the sheath is light reddish pigmented.
The mantle
The mantle of Cardiomya cleryana is thin, transparent and formed by three marginal folds (inner, middle and outer), which are antero-ventrally unfused forming a large pedal gape occupying ~1/3 the length of the ventral margin. A single mantle fusion occurs in the middle of the ventral margin and is formed by union of the inner folds and the inner surfaces of the middle folds, that is, type B fusion of Yonge (Reference Yonge1948, Reference Yonge1982). Ciliated rejection tracts were not observed here. Except for the pedal gape, therefore, there are no unfused mantle areas, that is, there is no 4th pallial aperture, as in some anomalodesmatans (Morton, Reference Morton1981c).
A more detailed cross-section of the ventral mantle margin of Cardiomya cleryana is illustrated in Figure 5. Here, each lobe comprises an elongated inner fold (imf), a reduced middle fold (mmf) and a large outer fold (omf) – the latter containing a large haemocoelic space (ha). From the periostracal groove (pg) between the latter two folds arises an exceedingly thin periostracum (p). A well developed pallial retractor muscle (prm) is present and connects the mantle margin with the shell. Two different glandular regions are present in the ventral mantle margin along the entire length of the pedal gape. The first, mantle margin gland I (mmg I), is located dorsally and parallels the fibres of the pallial retractor musculature. Well developed, this gland stains dark blue and is thus basophilic. The second region, mantle margin gland II (mmg II) stains light blue and is located internally between inner and middle mantle folds. Both glands may be mucus secreting. In life, a large amount of mucus was observed around the region of the pedal gape during the re-burrowing process and may prevent sediment entering the infra-septal chamber via this large aperture.
The septum
A horizontal septum (se) is present in Cardiomya cleryana dividing the mantle cavity into infra and supraseptal chambers. The infraseptal chamber is capacious (Figure 6). The septum is long and wide, ~2.5–3.5 mm in length and 400–750 µm in width, and comprises longitudinal muscle bands. It is suspended in the mantle cavity by robust posterior and anterior septal retractor muscles (psm/asm) that are attached to the shell valves just above their respective adductor muscles and lie close to the minute pedal retractor muscles (pprm/arpm) (Figure 6). A pair of narrow inner longitudinal septal muscles are also present lateral to the pores and septal pedal gape (spg), and attach close to the anterior septal retractor muscle (Figure 6). Lateral septal retractor muscles are also present being more concentrated posteriorly. This lateral septal retractor musculature is formed by finely separated bundles of delicate muscles that extend tangentially from the posterior septal retractor muscles and to the left and right shell valves dorsal to the visceral mass.
In Cardiomya cleryana there is also an extra lateral septal retractor muscle attached close to the anterior end of the siphonal retractor muscles, as reported for some species of Cardiomya, Cuspidaria and Myonera by Allen & Morgan (Reference Allen and Morgan1981) and Morton (Reference Morton2015). Left and right halves of the septum are united by the septal membrane (sm), which separates anteriorly to create the siphonal gape (~1.5 mm in length) through which the foot can protrude. Unlike in Grippina coronata Machado & Passos, Reference Machado and Passos2015 (Spheniopsidae) (Morton et al., Reference Morton, Dinesen and Ockelmann2015b) no cilia were observed along the margin of this membrane. The septum is perforated ventrally by four pairs of pores (sep 1–4) more or less equally spaced and measuring ~250 µm in diameter (Figure 4I). Inside these pores are small internal lips (100–150 µm) similar to those observed in Cuspidaria parva Verril & Bush, 1898 by Allen & Morgan (Reference Allen and Morgan1981, figure 17). These lightly muscular lips are ciliated dorsally forming a ring. A ring of such cilia (ci) are illustrated in Figure 4J.
The foot and byssus
The visceral mass is situated above the septum and is continuous with an anterior foot that extends through a septal gape in the septum anteriorly and can also be extended outwards from the similarly anterior pedal gape (pga) in the mantle margin (mm) to effect burrowing. The foot (f) is large and during the digging process can reach a length that almost equals the length of the shell. On average, living individuals each had a foot about 4–5 mm in length (Figure 7E). In fixed individuals, the foot is contracted (0.5–1.5 mm), anteriorly pointed with a narrow sole and a defined heel. In SEM, cilia can be seen to cover the ventral surface of the foot. Ventrally too, the sole has a deep pedal groove (peg) extending from the end of the heel to almost the tip of the foot (Figure 7F, G). No cilia occur on the dorsal surface of the foot.
The foot of Cardiomya cleryana is invaded by two pairs of pedal retractor muscles. These are a shorter anterior pair attached to the shell valves above the anterior adductor muscle. The posterior pedal retractor muscles are long, bifurcated at two points, and attach to the shell close to the posterior adductor muscle. The first bifurcation occurs in the middle region of the muscles fusing with the visceral mass. The second bifurcation occurs before the attachment to the shell valves, creating two small insertion points. A pair of short, fine, muscle bundles from the visceral mass usually attach to the gonads and merge with the posterior pedal retractor muscles just above the first bifurcation.
Internally, through sagittal and parasagittal sections it is possible to see the byssal gland (bg) that is formed by two glandular regions. The first of these is larger (bg I), wide and well developed throughout the length of the foot. The second is smaller (bg II) and restricted to the anterior region, close to the tip of the foot (Figure 7H). Posteriorly, the developed glandular region (bg I) opens via about 17 small ducts which converge and secrete a single long byssal thread (bt) (Figure 7H). In almost all individuals such a transparent thread was apparent and which in some could measure up to 20 mm in length, that is, three to four times the length of shell (Figures 6, 8 & 9C; see video in Supplementary material). This long byssal thread demonstrates that the foot must extend to an equal length so that it can plant it within the sediment (Figure 8) thereby securely anchoring each individual. During planting of the thread, the foot also twists to facilitate the necessary sediment probing action (Figure 8, f (tw)).
The alimentary system
The alimentary system of C. cleryana, as seen from the right side, is illustrated in Figure 6. Anteriorly, the septum ends at a large mouth (m) (~800 µm in diameter), which, in turn, has lateral muscular extensions forming the walls of the buccal funnel (Figure 9A & B). Small labial palps are present. The anterior palps (ap) are narrow, horn-shaped and measure ~400 µm in length. The posterior palps (pp) are reduced, flattened (~300 µm in length) and are located along the postero-lateral margins of the mouth (Figure 9A, B). The palps are ciliated and similar in shape to type I described by Allen & Morgan (Reference Allen and Morgan1981, figure 7). From the mouth a short, thick and muscular oesophagus (o) (~340 µm in diameter) opens into the anterior-dorsal part of the elongated-oval stomach (st) (Figure 9A, C). The stomach (type II of Purchon, Reference Purchon1956) is large (1.5–2.1 mm in length), thick, with a well defined posterior extension (pest) and a reduced crystalline style sac (css) (Figure 6). Laterally, the stomach is lined by an epithelium that secretes an internal thick layer of cuticle, the gastric shield (gs) (Figure 9A). Ventral and antero-ventral portions of the stomach are laterally covered by the digestive diverticulae (dd) while the posteroventral region is laterally covered by the gonads (g/te) (Figure 9A). The dorsal area of the stomach is not covered. In immature individuals, the stomach is surrounded by the digestive diverticulae.
In all individuals of C. cleryana examined, the stomach always contained ingested prey (ipi), either whole or partially digested (Figure 9A). In section, the sizes of these prey items ranged from 150–250 µm in length and most resembled small copepods (Crustacea) (Figure 9A). Sorting areas were also observed on the stomach floor. From the right side of the stomach floor arises the small crystalline style sac (css) (~350 µm in length) and the mid gut (mg), close to each other but separated other than at their origins with the stomach (Figures 6 & 9C), similar to C. knudseni (Allen & Morgan Reference Allen and Morgan1981, figure 32). The style-sac of C. cleryana is a short oval-circular cavity (~400 µm in diameter) inside which is a cylindrical style (~150–200 µm in diameter) (Figures 6 & 9C). Close to the style-sac opening into the stomach are two typhlosoles, one smaller the other larger.
The mid gut (mg) begins on the right side of the stomach floor, extends ventrally from the base of the style-sac and travels dorsally along the border of the right lateral wall of the stomach. Here it curves posteriorly and then its lumen expands and eventually gives rise to the hind gut (hg) (Figures 6 & 9C). In sagittal section, the mid gut is large and contains many skeletal remains of digested prey (ipi) (Figure 9A). Within the visceral mass, the digestive diverticulae (dd) are located anterodorsally. In living individuals, the rectum (rc), anus (an) and visceral ganglia (vg) are displaced posterior to the posterior adductor muscle when the siphons are extended (Figure 6).
The hind gut (hg) is straight, long and narrow eventually giving rise to the rectum (rc). Smaller fragments of prey skeletons occur inside the hind gut. The hind gut penetrates the pericardial cavity/heart (pc/h) traversing the ventricle of the heart from front to back (Figures 6 & 9A). In living individuals, with siphons extended, the rectum and the anus (an) are displaced by about 1 mm from the posterior end of the posterior adductor muscle (Figures 6 & 9D). The anus opens between the visceral ganglia (vg), releasing the faeces into the exhalant chamber and siphon.
The reproductive system
All sectioned specimens were either males or females, none was obviously hermaphroditic, and C. cleryana is, thus, possibly dioecious. However, only three individuals were sectioned and the smallest of these (5.2 mm shell length) was a male. The other two larger individuals (5.5 and 6.3 mm shell length) were both females, raising the possibility of protandric hermaphroditism as in C. costellata (Morton, Reference Morton2015). Moreover, the sex ratio was biased in favour of males (2:1) and since no individuals of a shell length <5 mm were collected, the expression of sexuality in C. cleryana remains speculative.
The gonads, testes (te) and ovaries (ov) of C. cleryana are located near the posterolateral and dorsal walls of the stomach and occupy a large part of the visceral mass (vm) (Figure 7A & B). In some cases, the gonads extend around almost all the organs of the visceral mass, and are evaginated as lateral pouches into the supraseptal chamber. The gonads of C. cleryana are formed by several follicular lobes. These lobes each have a small duct that connects to a wider one, the gonoduct. This opens into the posterodorsal region of the supraseptal chamber. The follicular lobes vary in size and shape depending on the individual. In living individuals, males and females can be identified by the shape and colour of their follicular lobes. The testes are usually thinner (0.1–0.4 mm) and whitish whereas the ovarian lobes are thicker (0.7–1.0 mm) with a transparent outer wall and containing small pink spheres – the oocytes (Figure 7A & B).
Although less common than males, it was possible to observe female gametogenesis, which in C. cleryana comprises oocytes in four stages of maturity: (i) immature oocytes (io) located in the wall of the ovarian follicle are the smallest (5–10 µm in diameter) with an oval to flattened shape. Each follicle may contain 70–100 immature oocytes; (ii) early maturing oocytes (emo) are also located in the walls of follicles (fw) close to the immature cells but are larger (20–30 µm in diameter) with a rounded shape and a large central nucleus; (iii) pre-vitellogenic, teardrop shaped, oocytes (pvo) (50–110 µm in diameter) are the most common cells in mature individuals and are always attached to the follicular wall via a stalk. Within the follicles are many (iv) mature oocytes (mo) (80–150 µm in diameter), which are characterized by a large amount of yolk displacing the nucleus to the cell periphery. This cell type was not attached to the follicle wall and was free in the lumen (Figure 7C). The testes could be differentiated into spermatogonia (spo), spermatocytes (spm) and spermatozoa (spz) (Figure 7D).
The nervous system
The nervous system of C. cleryana is similar to that described for most species of Cuspidariidae, with three pairs of ganglia: the cerebropleural, pedal and visceral. The cerebropleural (cg) pair is the largest with each ganglion measuring ~80 µm in diameter. These are located close to the anterior adductor muscle and dorso-laterally to the mouth. This pair is formed by elaborated nerve connections and each ganglion is formed by six branches: (i) a large commissure nerve (cn) (~800 µm in length) linking the pair; (ii) two divergent nerves (~350 µm in length), a labial nerve (ln) and a buccal nerve (bn) innervating the anterior labial palps and mouth; (iii) the dorsal adductor muscle nerve (an), inserted ventrally in the anterior adductor muscle. In C. cleryana the anterior adductor muscle is divided somewhat and has a thin groove in the middle; (iv) a muscular nerve (mmn), innervating the anterior septal and pedal retractor muscles with some of these nerve bundles also extending towards the mantle margin, and a visceral nerve (vn) extending into the visceral mass (Figure 9E).
The pedal ganglia (pega) (diameter ~150 µm) are located in the dorsal region of the foot near the ventral margin of the visceral mass and anterior to the byssal glands. Closely applied to the dorsal surface of the pedal ganglia is a pair of separate statocysts (sta) (~60 µm in diameter) (Figures 7I & 10). The statocysts comprise a small capsule of only 6 or 8 cells in transverse section and inside each capsule are numerous crystal-like statoconia (stc). The number (20–30) and size (10–15 µm in diameter) of these statoconia differ in each capsule. There is no statolith. The statocysts of C. cleryana resemble statocyst types B and C described by Morton (Reference Morton1985b, figure 3a, b). A similar situation was also observed for Spheniopsis brasiliensis Machado & Passos, 2016 (Spheniopsidae) (Morton et al., Reference Morton, Machado and Passos2015c).
When the siphons are contracted the visceral ganglia (vg) (~100 µm in diameter) of C. cleryana are situated beneath the posterior adductor muscle. In individuals with relaxed siphons, the ganglia are displaced by ~1 mm behind the posterior adductor muscle (Figures 6 & 9D). In both positions, the visceral ganglia are situated close to the anus and the base of the exhalant siphon. The same situation also applies to the rectum and the anus of C. cleryana, linked as they are to the visceral ganglia (Figures 6 & 9D). Between five to seven nerve bundles (cnb) pass from the ganglia into the visceral mass and foot and the ventral margin of the posterior adductor muscle and rectum (Figure 9D). Small nerve fibres also innervate the siphons.
DISCUSSION
Anatomical remarks
According to Poutiers & Bernard (Reference Poutiers, Bernard and Bouchet1995), Coan et al. (Reference Coan, Valentich-Scott and Bernard2000) and Coan & Valentich-Scott (Reference Coan and Valentich-Scott2012) the genus Cardiomya possesses only two diagnostic anatomical features, that is: a septum perforated by four pairs of pores, and a small lateral septal retractor muscle attached close to the anterior end of the siphonal retractor muscles. The second feature is not, however, unique to this genus and has been described for species of Bathyneaera such as B. hadalis (Knudsen, Reference Knudsen1970) by Allen & Morgan (Reference Allen and Morgan1981, figure 34). Among the 30 individuals of C. cleryana herein examined, all possessed the two diagnostic features identified above, although as described other important behavioural and morphological characters have also been identified and will be discussed below.
Life orientation
Few species of the Cuspidariidae have ever been examined alive, with information about the behaviour of this carnivorous family restricted to the work of Yonge (Reference Yonge1928) on Cuspidaria rostrata and Cuspidaria cuspidata, Reid & Reid (Reference Reid and Reid1974) on C. rostrata and Cuspidaria obesa, Reid & Crosby (Reference Reid and Crosby1980) on Cardiomya planetica and Allen & Morgan (Reference Allen and Morgan1981) on C. cuspidata.
This study examined 10 living individuals of Cardiomya cleryana. The main difference found between C. cleryana and other Cuspidariidae species was the life orientation adopted by this bivalve in the sediment. Morton (Reference Morton1987, figure 13) postulated, based on the illustrations of Cardiomya planetica by Reid & Reid (Reference Reid and Reid1974), that members of the Cuspidariidae adopt a position approximately perpendicular to the sediment surface. The orientation adopted by C. cleryana in the sediment, however, is ~45° degrees to the sediment surface (Figures 2 & 8).
The siphons
The siphons of Cardiomya cleryana are similar to those described by Yonge (Reference Yonge1928), Reid & Reid (Reference Reid and Reid1974), Allen & Morgan (Reference Allen and Morgan1981) and Morton (Reference Morton2015) for Cuspidaria rostrata, Cuspidaria cuspidata, Cuspidaria obesa and Cardiomya costellata, respectively. The presence of finger-shaped siphonal tentacles with micro papillae on the surface, each tipped with a pit occupied by sensory cilia, as well as two sets of inter-tentacular projections between the exhalant siphonal tentacles, differentiates C. cleryana from the above species. The siphonal tentacles of Cuspidata cuspidata and Cuspidaria obesa are club-shaped at their tips, whereas in Cuspidaria rostrata they are more elaborately expanded at the tips into foliate structures, each of which has two notches (Reid & Reid, Reference Reid and Reid1974, figure 2), and are thus different from the rounded structures seen in C. cleryana (Figure 4E & F).
The presence of siphonal tentacles (=siphonal papillae) with a ciliated apical pit have also been observed in Cardiomya planetica (Cuspidariidae) by Reid & Crosby (Reference Reid and Crosby1980, figure 5); Multitentacula venusta Krylova, Reference Krylova1995 (Protocuspidariidae) Krylova (Reference Krylova1995, figure 3D); Grippina coronata and Spheniopsis brasiliensis (Spheniopsidae) Morton et al. (Reference Morton, Machado and Passos2015b, Reference Morton, Machado and Passosc) and more recently in C. costellata (Morton, Reference Morton2015, figures 6–7). According to Reid & Crosby (Reference Reid and Crosby1980) and Morton et al. (Reference Morton, Machado and Passos2015b) these apical cilia are used as mechanoreceptors of the vicinal movements of potential prey. The same function may also be attributed to those of C. cleryana.
The colour pattern of the siphons of Cardiomya cleryana (orange red siphonal sheath and small white spots on the surface of the inhalant siphon and siphonal tentacles) has also been observed in other anomalodesmatans including Lyonsia californica Conrad, 1837 (black spots on both siphons and tentacles), Pandora filosa (brownish pigment spots on both siphons and tentacles), Cuspidaria cuspidata (scarlet pigments on the inhalant siphon and its tentacles) and both C. obesa and Cardiomya costellata (siphons coloured red in life) by Narchi (Reference Narchi1968, figure 2), Thomas (Reference Thomas1994, figure 3), Allen & Morgan (Reference Allen and Morgan1981, p. 529), Yonge (Reference Yonge1928) and Martins et al. (Reference Martins, Ávila, Borges, Madeira, Morton and Martins2009), respectively. The red end of the spectrum is lost quickly with depth so that it appears black, and is thus characteristic of many deeper water species. It is thus not unusual to see it in deeper water cuspidariids and their relatives.
The relationship with Bathyneaera
Similar structures to the exhalant inter-tentacular projections observed in Cardiomya cleryana have been reported previously in the literature by Knudsen (Reference Knudsen1970) and Reid & Crosby (Reference Reid and Crosby1980) for Myonera mexicana Knudsen, Reference Knudsen1970 and Cardiomya planetica (Dall, 1908), respectively, and thereafter by Allen & Morgan (Reference Allen and Morgan1981, figure 34B) and Krylova (Reference Krylova1993, figures 1 & 2) for species of Myonera and Bathyneaera, again respectively. According to Knudsen (Reference Knudsen1970) and Allen & Morgan (Reference Allen and Morgan1981), M. mexicana, Myonera demistriata (Allen & Morgan, Reference Allen and Morgan1981) (=Bathyneaera hadalis Knudsen, Reference Knudsen1970) and Myonera garretti Dall, 1908 have an ‘inter-tentacular tuberculous web’ along the free edge of the exhalant tentacles. Reid & Crosby (Reference Reid and Crosby1980, figure 3B) also observed ‘minor tentacles arising between the three exhalant siphonal tentacles and exhalant siphon’ in C. planetica. Later, Krylova (Reference Krylova1993) observed this same feature, now called ‘projections between the dorsal exhalant tentacles’ in Bathyneaera hadalis, B. tillamookensis (Dall, 1916), B. paleifera Krylova, Reference Krylova1993, B. disa (Bernard, Reference Bernard1989) and B. bernardi Krylova, Reference Krylova1993. Subsequently, this feature has been included in the diagnosis for species of Bathyneaera. The presence of these inter-tentacular projections in C. cleryana further adds to the arguments for a close relationship between Cardiomya and Bathyneaera corroborating the initial observations made by Scarlato & Starogobatov (Reference Scarlato and Starogobatov1983) and Krylova (Reference Krylova1993).
The function of these inter-tentacular projections remains uncertain however. Histological examination of Cardiomya cleryana suggests that the origin of the small papillae that form these projections is the same as that of the siphonal tentacles, that is, they originate on the middle (typically sensory) mantle fold. In addition, observations on living individuals also showed that these projections assist in closing the exhalant opening when the siphons are retracted. It is therefore suggested that the inter-tentacular projections function as: extra sensory structures assisting the siphonal tentacles in detecting potential prey; replacing damaged siphonal tentacles; and assist in the closing of the exhalant opening preventing unwanted particles from entering the supraseptal chamber.
In addition, the shape of the Cardiomya cleryana stomach is similar to the description given by Temkin & Strong (Reference Temkin and Strong2013) of that of Bathyneaera demistriata. In both species, an expansion of the stomach wall is evident. This expansion is only to the posterior region of the stomach in C. cleryana that is fused to the posterior pedal retractor muscle whereas in B. demistriata the stomach is anteroposteriorly expanded.
According to Krylova (Reference Krylova1993), the main differences between Cardiomya and Bathyneaera include: (i) occurrence depth – Cardiomya 0–4.000 m, Bathyneaera 439–8.430 m; (ii) the absence of a lateral tooth in the hinge plate; and (iii) a reduced rostrum in Bathyneaera species. In addition, (iv) the tips of the siphonal tentacles of C. cleryana but not Bathyneaera spp. have an apical ciliated pit and micro-papillae on their surface (this study).
The foot and byssal thread
Other new morphological features concerning Cardiomya cleryana and reported upon in this study include the presence of a long byssal thread and a greatly extensible foot (Figures 7E & 8). Although byssal threads have been reported upon for the representatives of a number of anomalodesmatan families such as the Lyonsiidae (Entodesma saxicola (Baird, 1863) =E. navicula (A. Adams & Reeve, 1850)), Lyonsiellidae (Lyonsiella horrida Allen & Turner, Reference Allen and Turner1974, Lyonsiella frielei Allen & Turner, Reference Allen and Turner1974 and Policordia jeffreysi (Friele, 1879)), Verticordiidae (Verticordia triangularis Locard, 1898, Verticordia quadrata Smith, 1885 and Spinosipella deshayesiana (P. Fisher, 1862)) and, more recently, the Poromyidae (Dillema frumarkernorum Leal, Reference Leal2008 and Dillema spectralis Leal, 2008) (Yonge, Reference Yonge1952; Allen & Turner, Reference Allen and Turner1974; Morgan & Allen, Reference Morgan and Allen1976; Leal, 2008; Simone & Cunha, Reference Simone and Cunha2008;) such a long thread seen in C. cleryana has never been reported upon before for any anomalodesmatan.
More specifically, with regard to the Cuspidariidae, Allen & Morgan (Reference Allen and Morgan1981, p. 440) reported that ‘Threads have been seen in only two species’ but did not identify which taxa nor illustrate them. Krylova (Reference Krylova1993) reported upon the presence of a byssal thread in a single individual of Bathyneaera tillamokensis (Cuspidariidae) but also did not either describe or illustrate this feature. The byssal thread identified in Cardiomya cleryana seems to be an important adaptation to survival in an area of the seabed where there are strong seabed currents (Dottori et al., Reference Dottori, Siegle and Castro2015). In small Araçá Bay, strong currents (South Atlantic Central Water) constantly cause the sediment to be displaced from the seabed and they thereby modify the benthic environment and displace unsecurely anchored species. The long byssal thread thus favours survival in such a disturbed habitat.
A similarly long foot has, however, been described for species of the Mytilidae such as Modiolarca subpicta (Cantraine, 1835) (=Musculus subpictus Cantraine, 1835) and is similarly responsible for the planting of byssal threads in the juvenile (Morton & Dinesen, Reference Morton and Dinesen2011). Another example is provided by Crenella decussata (Montagu, 1808) which has a long foot mainly used to construct an adventitious tube within which an individual resides (Morton et al., Reference Morton, Dinesen and Ockelmann2015a). Some representatives of the Lucinoidea such as Diplodonta punctata (Say, 1822) (Domaneschi, Reference Domaneschi1979), Lucina pectinata (Gmelin, 1791) = Phacoides pectinatus (Gmelin, 1791) (Narchi & Assis, Reference Narchi and Assis1980), Anodontia philippiana (Reeve, 1850) =Pegophysema philippiana (Reeve, 1850) (Taylor & Glover, Reference Taylor and Glover2006, figure 4) also have a long, highly extensible, foot. This is also the case for some thyasirids such as those reported by Payne & Allen (Reference Payne and Allen1991), Dufour & Felbeck (Reference Dufour and Felbeck2003) and Passos et al. (Reference Passos, Meserani and Gros2007).
The statocysts
The nervous system of Cardiomya cleryana is similar to that described by Allen & Morgan (Reference Allen and Morgan1981) for other species of the Cuspidariidae and is formed by three pairs of ganglia: cerebropleural, pedal and visceral. The position of the visceral ganglia in C. cleryana is similar to that observed in Cuspidaria rostrata by Reid & Reid (Reference Reid and Reid1974, figure 3) and Cardiomya planetica by Reid & Crosby (Reference Reid and Crosby1980, figure 4) where the ganglia are situated distant from the posterior adductor muscle (Figures 6 & 9D). They are, however, different from the situation observed in Cardiomya costellata by Morton (Reference Morton2015, figure 21A), where the ganglia are located beneath the posterior adductor muscle (but with the siphons retracted).
The only feature of the nervous system observed in Cardiomya cleryana that differs from other Cuspidariidae is the morphology of the statocysts. According to Morton (Reference Morton1985b), three types of statocyst occur in representatives of the Anomalodesmata: A, B and C. Type B can, however, be subdivided into three groups on the basis of differences in statolith structure. In C. cleryana the statocysts are closely applied to the dorsal surface of the pedal ganglia with each capsule formed by between 5–6 cells (in transverse section) and with a large number of statoconia in the capsule's cavity (Figures 7I & 10). This is similar in part to Morton's Type B2 seen in Parilimya maoria Dell (1963) (Parilimyiidae) (statocysts closely applied to the pedal ganglia with both a statolith and statoconia inside) and to Type C, exclusive to the Cuspidariidae. This latter type is formed by 4–5 cells, each with little cytoplasm. Spheniopsis brasiliensis (Spheniopsidae) also has statocsyts which resemble both Type B1 and the Type C; although Morton et al. (Reference Morton, Machado and Passos2015c) defined them as Type C. The presence of many crystalline statoconia in Cardiomya cleryana suggests that though a sedentary lie-in-wait predator, it is capable of fine orientation within the sediment as demonstrated in this study.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at http://dx.doi.org/10.1017/S0025315416000564.
ACKNOWLEDGEMENTS
Thanks are due to Prof. Dra. A.C.Z. Amaral (DBA/IB/UNICAMP) for her permission to use microscopy facilities; to Dr E. Strong (USNM) who provided the photographs of some species of Cuspidariidae; to CEBIMar-USP for logistic support; to Dr A. Migotto and T. Forroni MSc for assistance during the filming activities; to A.C.S. Sprogis and S.M.F. Ferraz (Laboratory of Microscopy – IB/UNICAMP), for providing microscopy assistance and all those who participated in the BIOTA/FAPESP-Araçá Project.
FINANCIAL SUPPORT
This research was partly funded by a grant awarded to F. M. Machado by CAPES and by FAPESP, in association with the Project BIOTA/FAPESP-Araçá (Process no. 2011/50317-5).












