INTRODUCTION
The demersal fish family Liparidae has over 350 species in the world. They are widely distributed from shallow to deep waters, including the hadal zone which is >7000 m deep (Jamieson et al., Reference Jamieson, Fujii, Solan, Matsumoto, Bagley and Priede2009). Although some phylogenetic (Knudsen et al., Reference Knudsen, Møller and Gravlund2007; Chernova, Reference Chernova2008) and behavioural studies (Able & Musick, Reference Able and Musick1976; Stein et al., Reference Stein, Drazen, Schlining, Barry and Kuhnz2006) have been undertaken, their life histories are still poorly understood.
Liparis tanakae (often noted as L. tanakai) is a common snailfish species in the coastal waters of Japan, Korea, and China (Chen et al., Reference Chen, Liu, Zeng and Su1997; Huh, Reference Huh1997; Rhodes, Reference Rhodes1998; Jin et al., Reference Jin, Xu and Tang2003); the genus Liparis is a shallow-water group (Knudsen et al., Reference Knudsen, Møller and Gravlund2007). In some localities, the snailfish is commercialized. From 2004 to 2010, the amount of this species caught by trawls and gill-nets, and landed at Soma-Haragama Fish Market (37°50′N 140°58′E), Japan, ranged from 16 to 27 tons (Fukushima Prefecture, unpublished data, 2011). The snailfish have a lifespan of one year and grow up to 300 mm in body length (Kawasaki et al., Reference Kawasaki, Hashimoto, Honda and Otake1983). They are a dominant species at a depth of 138 m off Sendai Bay (38°N 141°E) in early November (Fujita et al., Reference Fujita, Inada and Ishito1995a). Juveniles and adults feed chiefly on shrimps and fish (Kawasaki et al., Reference Kawasaki, Hashimoto, Honda and Otake1983; Honda, Reference Honda1985; Kobayashi & Hiyama, Reference Kobayashi and Hiyama1991), while the larvae feed on copepods (Plaza-Pasten et al., Reference Plaza-Pasten, Katayama, Nagashima and Omori2002).
Recently, the snailfish has been recognized as a predator for both wild and hatchery-reared (HR) Japanese flounder Paralichthys olivaceus (Tomiyama et al., Reference Tomiyama, Ebe, Kawata and Fujii2009). Japanese flounder is a commercially important flatfish and is a major target species for stock enhancement in Japan. In Fukushima, Japan, the HR juveniles around 100 mm are released annually in coastal waters <10 m deep from June to November (Tomiyama et al., Reference Tomiyama, Watanabe and Fujita2008), but the stocking effectiveness varied from year to year. This variation probably reflected the variation in the survival of HR fish after release. Post-release predation of HR fish is the major mortality factor for released juveniles (Sudo et al., Reference Sudo, Kajihara and Fujii2008). To reduce the amount of predation, the habitat use and feeding of predators must be carefully considered.
This study aims to reveal when and where the predation by the snailfish on Japanese flounder can occur. To reveal the habitat overlap between the snailfish and Japanese flounder, we investigated the seasonal and spatial patterns in the bathymetric distribution of both species. In addition, the size relationship between the two species was explored as well as the ontogenetic change in the feeding habits of snailfish to assess the possibility of predation. Based on these results, we suggest the optimal releasing season for HR Japanese flounder to forestall predation by snailfish.
MATERIALS AND METHODS
Study sites and sample collection
Monthly surveys at latitude of 37°00′N off Fukushima, Japan were conducted. Additional samples of the snailfish for stomach contents analysis were collected at latitudes of 36°50′N and 37°50′N. Substrates are mostly sand or muddy-sand at all sampling locations.
To investigate seasonal variation in the abundance of snailfish and 0-year-old Japanese flounder, monthly daytime collections with three gears were conducted from February 2004 to December 2006. First, a beam trawl (2 m wide and 3 mm mesh) was towed by the RV ‘Takusui’ (30 tons) at a speed of 2 knots for 15 min (~ 0.9 km) at depths of 7 m and 15 m. Second, an otter-trawl net (~ 7.5 m mouth opening, 13 m long and 10 mm mesh) was towed by the RV ‘Takusui’ at a speed of 2.5 knots for 30 min (~ 2.3 km) at depths of 10, 20, 30 and 50 m. Third, a larger otter trawl net (~ 14 m mouth opening, 40 m long and 27 mm mesh) was towed by the RV ‘Iwaki-maru’ (159 tons) at a speed of 3 knots for 30 min (~ 2.8 km) at depths of 100, 125, 150, 175 and 300 m.
All samples were selected on-board after collection and brought to the laboratory in a chilled condition.
Laboratory observation and data analyses
Total length (TL, mm) of the snailfish and 0-year-old Japanese flounder collected by the beam trawl and otter trawls were measured. Stomachs of the snailfish were dissected and stomach contents were identified according to species or the lowest possible level for each prey item; the number and wet weight of each prey item were recorded.
The density of both species (the number of fish collected per 1000 m2) was determined for each depth, each month and each year. Catch efficiency was not considered. Towing distance was calculated from a Differential Global Positioning System.
To test if the larger snailfish individuals inhabit deeper areas, we constructed linear mixed models (LMM) in which the TL of the snailfish was used as the response variable. Month (categorical data) and depth (continuous data) were used as the initial explanatory variables. Year was included as a random factor. The model was finalized by stepwise backward selection based on the Akaike information criterion (AIC). The LMM was constructed for the data taken from March to September and October to December, as the snailfish move to deeper water in the former period and back to shallow areas in the latter period (see Results). The beam trawl samples were not used for this modelling because of low catchability for larger individuals by this gear. The modelling was conducted using the software R 2.13.0 with the package lme4 (Bates et al., Reference Bates, Maechler and Bolker2011).
To assess the TL of 0-year-old Japanese flounder vulnerable to the snailfish, the size relationship was derived from a previous study (Tomiyama et al., Reference Tomiyama, Ebe, Kawata and Fujii2009), as follows:
where TLmax is the maximum TL of Japanese flounder vulnerable to the snailfish of TLs. In this relationship, the mouth gape of the snailfish is assumed to be equal to the maximum body depth of Japanese flounder. Using this equation, we estimated the maximum TL of Japanese flounder vulnerable to the snailfish for each month. The average TL of snailfish collected at depths of ≤50 m in each month was used as TLs. The proportion of Japanese flounder vulnerable to the snailfish in each month was estimated from the size distribution of Japanese flounder.
To identify the important prey for the snailfish, the index of relative importance (%IRI) of each prey item was determined as follows:
where N, W, and F represent the number of prey consumed, the weight of prey consumed, and the frequency of consuming the prey, respectively. The %IRI was determined for each size group of ≤99 mm, 100–199 mm and ≥200 mm TL. To describe the ontogenetic diet shift of snailfish, their prey items were separated into six categories: gammarid, krill, natantian decapod, other crustacean, fish and others. The %IRI was determined for each size group (every 50 mm).
RESULTS
Bathymetric distribution and growth
The snailfish were collected from February at depths of ≤15 m (Figure 1). They were observed at depths around 10 m until March. Then, they were collected at depths of 7–50 m from April to June. They were seldom observed at depths of ≤50 m from August to October. On the contrary, many individuals were observed at depths of 100–200 m from July to October. No snailfish was collected at a depth of 300 m. The snailfish were often observed at depths around 10 m again from November to December.

Fig. 1. Seasonal changes in the density of Liparis tanakae and 0-year-old wild Paralichthys olivaceus. Monthly data were averaged among years from 2004 to 2006. Densities were investigated using (A) a 2 m beam trawl, (B) a small otter trawl and (C) an otter trawl. Catch efficiency was not taken into consideration. Solid and hatched bars denote L. tanakae and P. olivaceus, respectively.
Japanese flounder were observed from August at a depth of 10 m. They were observed at depths of ≤15 m until September. Thereafter, they were observed at depths of ≤50 m until December. No individual was collected at depths of ≥100 m.
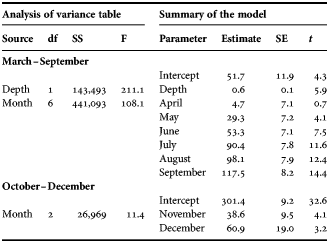
The TL of snailfish increased linearly as the season progressed (Figure 2). Individuals with <100 mm TL were not collected from July to December. The largest individual had 433 mm TL. The size of the snailfish tended to be larger for those collected at depths of ≥100 m than at depths of ≤50 m, especially in July. The depth was involved as an explanatory variable in the LMM for the snailfish TL, using the data from March to September, while that was excluded from the LMM using the data from October to December (Table 1); the model indicated that larger individuals inhabited deeper areas until September, but thereafter no relationship was found between depth and body size of the snailfish.

Fig. 2. Monthly changes in the total length of Liparis tanakae and 0-year-old wild Paralichthys olivaceus. The data were pooled among years and were shown by mean ± SD. Circles, triangles, and squares indicate L. tanakae caught at depths of ≤50 m, L. tanakae at depths of ≥100 m and 0-year-old wild P. olivaceus at depths of ≤50 m, respectively.
Table 1. Results of the linear mixed model for total length of the snailfish. Year was involved as a random factor. Depth was excluded from the model for October–December through model selection.
Habitat overlap between the snailfish and Japanese flounder was observed from September to December, although the snailfish were observed only rarely at depths ≤50 m during September and October. The maximum TL of Japanese flounder vulnerable to the snailfish of average size was estimated at 97 mm, 121 mm, 137 mm and 165 mm in September, October, November and December, respectively. The proportions of Japanese flounder smaller than these sizes were 77%, 44%, 48% and 78% in respective months (Figure 3).

Fig. 3. Length–frequency distributions of (A) Liparis tanakae and (B) 0-year-old wild Paralichthys olivaceus caught at depths of ≤50 m from September to December. The data were pooled among years (2004–2006). Solid bars in the right panels indicate individuals that are vulnerable to L. tanakae (average length at each month: 209 mm, 290 mm, 297 mm, and 357 mm total length in September, October November, and December, respectively).
Feeding of snailfish
The stomach contents of 794 snailfish samples, including 44 empty stomachs were examined. The diet of collected snailfish of ≥100 mm TL was chiefly natantian decapods and fish, while that of individuals of <100 mm TL was gammarids (Table 2). The sand shrimp Crangon spp. (mostly Crangon uritai) had the highest %IRI throughout the survey. Fish prey with the highest %IRI was H-spot eelpout Davidijordania poecilimon (Zoarcidae). Some commercially important fish, such as Pacific cod Gadus macrocephalus and flatfish (Japanese flounder, stone flounder Platichthys bicoloratus, littlemouth flounder Pseudopleuronectes herzensteini and red tongue sole Cynoglossus joyneri) were found in stomachs of snailfish.
Table 2. Percentage index of relative importance contributions of prey items to the overall diets of Liparis tanakae in each total length (TL) class in the study area. Numbers of fish that had food in their stomachs were 176, 279 and 268 for 19–99 mm, 100–199 mm, and ≥200 mm, respectively.

The diet of the snailfish shifted ontogenetically (Figure 4). Initial predominant prey for the snailfish of <50 mm was gammarids, while that for samples of ≥100 mm TL was natantian decapods. Fish were frequently consumed by snailfish of ≥150 mm TL. The proportion of fish was larger than that of natantian decapods for snailfish of ≥350 mm TL. Some prey species were consumed in specific depth ranges. Gammarids were consumed mostly by snailfish obtained at depths of ≤15 m, while krill were consumed only by individuals at depths of ≥100 m. The sand shrimp were consumed at all depths. H-spot eelpout were consumed at depths of ≥50 m. Pacific cod were consumed by snailfish collected at depths of 20–100 m, while flatfish were mostly consumed at depths of ≤30 m. Red tongue sole were frequently consumed only in December 2006.

Fig. 4. The index of relative importance (IRI) of stomach contents of Liparis tanakae at each total length (TL) class. N indicates the number of fish examined except those with empty stomach.
Five Japanese flounder were observed from four individual snailfish collected at depths of <10 m from September to December. Of the five individuals of Japanese flounder, three were wild and two were hatchery-reared fish, as determined from the hypermelanosis on the blind side of hatchery-reared fish. The consumed wild Japanese flounder had TLs ranging from 45 to 98 mm, while one of the consumed hatchery-reared fish had a TL of 124 mm.
DISCUSSION
The present study clearly revealed the seasonal migration of snailfish: they migrated to deep areas at depths of ≥100 m from July to October, and then they were back to shallow areas from November to December. The migration to shallow areas from November to December is probably related to their spawning, since their spawning season is considered to be from December to January around Sendai Bay (Kawasaki et al., Reference Kawasaki, Hashimoto, Honda and Otake1983). The encounter probability between the snailfish and 0-year-old Japanese flounder would be relatively high at depths around 10 m during November to December, although Japanese flounder expand their habitat to depths of ≤50 m from October to December.
The summer migration of the snailfish to deeper areas seems to be related to their preferable temperature conditions. A similar migration pattern in relation to avoidance of high water temperature was observed in the congeneric species Liparis liparis (Henderson & Holmes, Reference Henderson and Holmes1990). Larger snailfish were thought to migrate earlier and inhabit deeper areas under lower temperature conditions, indicating the size-related temperature preference. Another possible mechanism explaining the migration of snailfish to deeper areas is the change in their food demand. However, this idea is unlikely because they chiefly consume sand shrimps that are abundant both in shallow and deep areas.
The growth of snailfish is extremely fast: males reach over 400 mm TL and weigh 1 kg at one year old (Kawasaki et al., Reference Kawasaki, Hashimoto, Honda and Otake1983). It could be the fastest growth among demersal fish, although some pelagic fish, such as dolphinfish, achieve a large size of more than 600 mm fork length at one year old (Schwenke & Buckel, Reference Schwenke and Buckel2008).
The rapid growth of snailfish and their ontogenetic shift to piscivory may indicate that the risk of predation on flatfish increases as the snailfish grow. The snailfish has been reported as a piscivore (Fujita et al., Reference Fujita, Kitagawa, Okuyama, Ishito, Inada and Jin1995b). However, flatfish were not frequently observed from the snailfish stomachs, suggesting that the snailfish consume flatfish only occasionally. They feed mainly on Japanese sandlance in Sendai Bay (Kawasaki et al., Reference Kawasaki, Hashimoto, Honda and Otake1983), although we did not find sandlance in snailfish stomachs, which was possibly due to the absence of sandlance in the study site (Tomiyama & Kurita, Reference Tomiyama and Kurita2011). Instead, H-spot eelpout were chiefly consumed at depths of ≥50 m in the study area. On the other hand, the preference of the snailfish for sand shrimps is commonly observed at different localities (Kawasaki et al., Reference Kawasaki, Hashimoto, Honda and Otake1983; Kobayashi & Hiyama, Reference Kobayashi and Hiyama1991; Jin et al., Reference Jin, Zhang and Xue2010).
We estimated that more than 40% of 0-year-old Japanese flounder are vulnerable to the snailfish from September to December. This may be an overestimate, because the snailfish consumed Japanese flounder with relatively smaller sizes than they can capture (Tomiyama et al., Reference Tomiyama, Ebe, Kawata and Fujii2009). Although the snailfish grow rapidly, juvenile Japanese flounder also grow fast, at a rate of ~ 2 mm TL d−1 in their nursery ground (Tomiyama et al., Reference Tomiyama, Uehara and Kurita2013), suggesting that their risk of predation by the snailfish is not so high.
Compared to wild fish, hatchery-reared fish are more susceptible to predation, especially during the short period after their release (Sudo et al., Reference Sudo, Kajihara and Fujii2008). It was recorded that there was one snailfish (355 mm TL) which consumed eight individuals of HR Japanese flounder (64–114 mm TL) released at a depth of 6 m in November 2004 (Tomiyama et al., unpublished data), indicating that the snailfish can be a strong predator for HR Japanese flounder. To forestall predation by snailfish, it is essential not to release HR Japanese flounder during November and December, when large snailfish with higher piscivory appear again at shallow depths for spawning.
ACKNOWLEDGEMENTS
We thank Michio Sato and the crews of the RV ‘Takusui’ and RV ‘Iwaki-maru’ of Fukushima Prefectural Fisheries Experimental Station for their help in collecting samples. We also thank anonymous referees for their helpful comments on the manuscript.
FINANCIAL SUPPORT
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Supplementary materials and methods
The Supplementary material reffered to in this article can be found online at journals.cambridge.org/mbi.







