Article contents
Dynamic covalent hexahydrotriazine breakdown through nucleophilic attack by phosphine
Published online by Cambridge University Press: 27 March 2019
Abstract
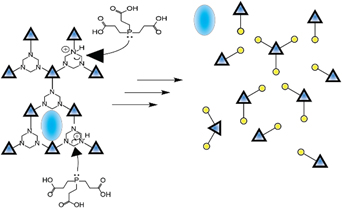
In the current manuscript we discuss the response of dynamic metallogels that display reversion to the liquid state when exposed to phosphines. The metallogels are formed through the condensation of formaldehyde and poly(alkyloxide)amines in polar aprotic solvents. The gel formation can be catalyzed with trivalent metals (Al(III and Fe(III)) with concomitant enhanced dynamism (gelation/degelation). When various phosphines are introduced, the metallogel is irreversibly liquefied. This process adds a new vector for controlling the bulk properties of this class of materials. Here, we explore the mechanism in detail for the reaction of tris(carboxyethyl)phosphine with N,N,N-triethoxylethyl-1,3,5-hexahydro-1,3,5-triazine (HEHT, 1) a stable derivative of the active hexahydrotriazine (HT) core in dimethylformamide in the presence or absence of Al(III). Additionally, density functional theory is used on the model N,N,N-trimethyl system (MHT, 2) to estimate reaction parameters and predict nuclear magnetic resonance spectra.
Information
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2019
References
- 1
- Cited by

