Article contents
Monolithic quartz platform for cellular contact guidance
Published online by Cambridge University Press: 16 March 2020
Abstract
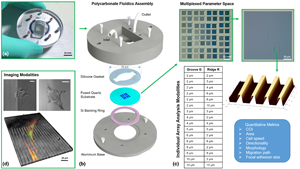
Contact guidance is vital to many physiological processes, yet is still poorly understood. This is partly due to the variability of experimental platforms, making comparisons difficult. To combat this, a multiplexed approach was used to fabricate topographical cues on single quartz coverslips for high-throughput screening. Furthermore, this method offers control of surface roughness and protein adsorption characterization, two critical aspects to the in vitro environment often overlooked in contact guidance platforms. The quartz surface can be regenerated, is compatible with versatile microscopy modes, and can scale up for manufacturing offering a novel platform that could serve as a potential standard assay.
Information
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2020
References
- 3
- Cited by

