Published online by Cambridge University Press: 10 July 2017
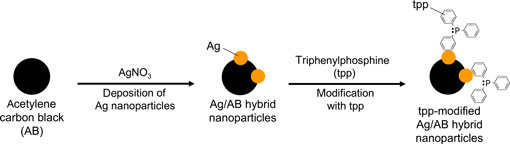
Here we report a doping method based on charge transfer interaction for an easily obtainable carbon material, carbon black (acetylene carbon black and Ketjenblack), as a main raw material. The n-type doping of those carbon blacks, generally p-type material, was conducted with a molecular dopant, triphenylphosphine (tpp). The key was to modify the surface of carbon blacks with silver (Ag) nanoparticles to attach tpp molecules on the surface of Ag. Our method is expected to be used for the fabrication of functional devices (such as thermoelectric devices) from p- and n-type materials.