Article contents
Hydrogel-based microchannels to measure confinement- and stiffness-sensitive Yes-associated-protein activity in epithelial clusters
Published online by Cambridge University Press: 07 September 2017
Abstract
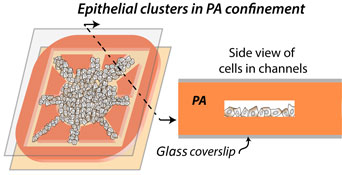
Nuclear translocation of Yes-associated-protein (YAP) in single cells serves as a key sensor of matrix stiffness. On two-dimensional (2D) polyacrylamide (PA) hydrogels, we found that nuclear YAP localization in epithelial clusters increases with gel stiffness and reduces with cell density. To measure YAP activity in 3D-like confinement of tunable stiffness, we fabricated PA-based microchannels. Here, narrower channels enhanced nuclear YAP localization even in softer extracellular matrix and denser epithelial clusters, both of which reduced YAP activation in 2D. Thus, the presented hydrogel microchannel-based platform may reveal new mechanosensitive cellular signatures in 3D-like settings, which cannot be captured on standard 2D hydrogels.
Information
- Type
- Biomaterials for 3D Cell Biology Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2017
References
- 8
- Cited by

