Article contents
Molecular valves for colloidal growth of nanocrystal quantum dots: effect of precursor decomposition and intermediate species
Published online by Cambridge University Press: 16 July 2018
Abstract
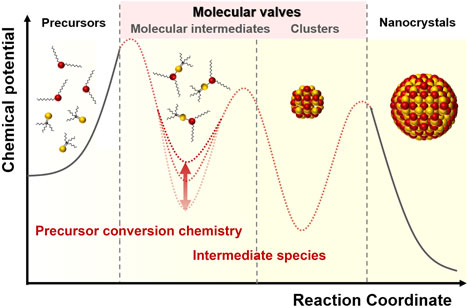
The ability to manipulate matter on the nanometer length scale is an important scientific goal, and the progress in the field of colloidal nanocrystal (NC) growth in the past decades has opened avenue for controlled synthesis of nanoscale materials with many unique physical properties that could enhance existing technologies or give rise to entirely new technologic applications. At the center of the progress is ever-increasing understanding on molecular interactions within colloidal synthesis, in which nucleation and growth each plays a critical role in the control of size, shape, morphology, and structure of NCs. Semiconductor NCs in quantum confinement regime, referred to as quantum dots (QDs), highlight the importance of such control over geometric parameters, since QDs exhibit size- and shape-dependent optical properties. In this paper, we demonstrate important aspects that govern QDs growth in the context of (i) precursor conversion chemistry, and (ii) intermediate species including molecular complex and clusters. Advances in understanding the growth chemistry of QDs have proved the significance of how precursors decompose and produce intermediate species. We review recent progress in regards to the synthetic chemistry of colloidal QDs and discuss our perspective on challenges and promises in the controlled large-scale synthesis of QDs.
Information
- Type
- Prospective Articles
- Information
- Copyright
- Copyright © Materials Research Society 2018
References
- 4
- Cited by


