Introduction
CVD, which include CHD (myocardial infarction and angina), stroke and peripheral vascular disease( Reference Schwenke 1 ), are a key contributor to the burden of disease globally( 2 ). Over the past 50 years, the prevalence of CVD has fallen in Western populations; however, CVD are currently the major cause of death in women in the UK, accounting for 32 % of all deaths( 3 ). Furthermore, the prevalence of CVD is dramatically increasing in other areas, including Eastern Europe, Asia and the Indian subcontinent( 4 ).
The aetiology for CVD is multifactorial and includes several modifiable risk factors, such as cigarette smoking, a sedentary lifestyle, obesity, elevated blood pressure, dyslipidaemia, type 2 diabetes mellitus, and non-modifiable factors, such as advancing age, sex, family history of heart disease and ethnicity( Reference Libby, Ridker and Maseri 5 , Reference Smith 6 ). Among the non-modifiable risk factors, ageing is associated with progressive endothelial dysfunction (characterised by a loss of vascular wall homeostasis leading to a decrease in vascular reactivity and raised blood pressure) in both sexes, although it appears to occur earlier in men than women( Reference Celermajer, Sorensen and Spiegelhalter 7 ). The most prominent sex-related difference in physiological ageing is the menopause (cessation of menstruation) in women, which usually occurs between the ages of 45 and 55 years, with 51 years being the average age of menopause in the UK( Reference Pokoradi, Iversen and Hannaford 8 ). This natural part of ageing in women contributes a significant cardiovascular milestone in terms of both physiology and pathology since oestrogen deficiency is known to impair lipid metabolism and endothelial function, and the menopause is a recognised risk factor for CVD( Reference Wenger, Speroff and Packard 9 ). It has further been shown by van der Schouw et al. ( Reference Van der Schouw, van der Graaf and Steyerberg 10 ) that for each year of delay in the age of onset of the natural menopause, CVD risk falls by 2 %.
Diet is one of the most important modifiable risk factors in relation to CVD( Reference Yusuf, Hawken and Ôunpuu 11 ). As a strategy to reduce the incidence of CVD, public health policy makers recommend that intakes of dietary SFA are reduced to <10 % of total energy in the UK( 12 ). Substituting SFA with MUFA or PUFA may provide additional benefits in relation to CVD risk factors, including reductions in the fasting lipid profile and improvements in endothelial function. A systematic review proposed that lowering dietary SFA intake by modifying dietary fat composition, rather than reducing total fat intake, may reduce cardiovascular events by 14 %( Reference Hooper, Summerbell and Thompson 13 ). Since individuals spend a large proportion of the day in the fed (postprandial) state, modifications to the fatty acid composition of our meals that are repeated on a daily basis may have a significant impact on postprandial lipaemia and vascular health, which over time could affect CVD risk. Therefore, the aim was to systematically review and critically evaluate the existing evidence from acute studies comparing meals rich in SFA, MUFA and n-6 PUFA on postprandial lipaemia, vascular reactivity, blood pressure and biomarkers of vascular function and inflammation. We chose to specifically focus on postmenopausal women since they represent an understudied group within the population at increased CVD risk.
Before presentation of the methodology and results of the literature review, we provide a general overview of postprandial lipaemia and vascular function, and describe the individual impact of the menopause and dietary fat composition on these CVD risk factors.
Postprandial lipaemia
With the pattern of meal ingestion in Western societies, a greater part of the day is spent in the postprandial than fasting state. Kolovou et al.( Reference Kolovou, Mikhailidis and Nordestgaard 14 ) defined postprandial lipaemia as a complex syndrome characterised by non-fasting hypertriacylglycerolaemia and its augmentation is associated with an increased risk of cardiovascular events. Following a fat-containing meal, there is a transient rise in circulating TAG-rich lipoproteins (TRL), such as chylomicrons (CM) and VLDL. After entering the circulation, the CM-TAG is hydrolysed into NEFA by lipoprotein lipase (LPL), forming cholesteryl ester-rich CM remnants, which are cleared by the liver via receptor-mediated uptake. VLDL follows a similar route of metabolism in the circulation as CM particles, although VLDL are hydrolysed at a slower rate as the larger CM are the preferential substrate for LPL. VLDL TAG depletion produces smaller VLDL (intermediate-density lipoprotein or VLDL remnants), a proportion of which are metabolised to form LDL before they are cleared via hepatic LDL receptors using apo B-100 as a ligand. During the postprandial period, there is an accumulation of TRL in the circulation due to competition between intestinal and hepatic TRL for the same lipolytic and receptor-mediated uptake( Reference Bjorkegren, Packard and Hamsten 15 ). A delayed clearance of TRL in the circulation enhances the accumulation of TRL particles carrying acceptor sites for the cholesteryl ester transfer protein, which transfers TAG from TRL (CM and VLDL) and exchanges it with cholesteryl esters from HDL and LDL. Remodelling of the lipid content of the LDL and HDL particles make them suitable substrates for LPL and hepatic lipase, leading to the formation of smaller and denser LDL (LDL3) and HDL (HDL3) particles( Reference Jackson, Poppitt and Minihane 16 ). HDL3 is rapidly removed from the circulation, decreasing circulating HDL-cholesterol (HDL-C) concentrations, which is one proposed mechanism for the inverse association between exaggerated postprandial lipaemia and CVD risk( Reference Chapman, Le Goff and Guerin 17 ). Another possible mechanism is that LDL3 has a lower binding affinity for the LDL receptor, reducing their rate of clearance from the circulation and enabling them to infiltrate the arterial wall( Reference Jackson, Poppitt and Minihane 16 ).
Since atherosclerosis is now also considered to be a postprandial phenomenon, three large prospective cohort studies aimed to determine the link between cardiovascular events and non-fasting TAG( Reference Bansal, Buring and Rifai 18 – Reference Lindman, Veierød and Tverdal 20 ). In the Norwegian Counties Study, hazard ratios of 1·2 and 1·03 for deaths from CVD per 1 mmol/l increase in non-fasting TAG were reported in women and men, respectively, after 27 years of follow-up in a total of 86 261 participants( Reference Lindman, Veierød and Tverdal 20 ). Furthermore, the Copenhagen City Heart Study that followed 7581 women and 6391 men for 31 years showed that relative to women with non-fasting TAG of <1 mmol/l, hazard ratios for myocardial infarction ranged from 1·5 for women with TAG between 1·0 and 1·99 mmol/l rising to 4·2 for those with TAG ≥5 mmol/l( Reference Langsted, Freiberg and Tybjaerg‐Hansen 19 ). However, the corresponding hazard ratios for men were lower at 1·3 and 2·1, respectively. In the Women’s Health Study, fasting (n 20 118) and non-fasting (n 6391) TAG predicted cardiovascular events after 11·4 years of follow-up after adjusting for age, blood pressure, smoking status and hormone therapy. The authors also reported that the strongest association between cardiovascular events and non-fasting TAG occurred 2–4 h after the last meal, with the association declining as the fasting time increased( Reference Bansal, Buring and Rifai 18 ). These studies have demonstrated the greater importance of non-fasting than fasting TAG concentrations as a predictor of CVD risk in women compared with men.
The impact of menopausal status on the variability of the postprandial lipaemic responses has been reported in a number of studies( Reference van Beek, de Ruijter-Heijstek and Erkelens 21 – Reference Jackson, Abraham and Smith 24 ) (online Supplementary Table S1). In general, premenopausal women have lower postprandial TAG responses than men( Reference Cohn, McNamara and Cohn 25 – Reference Tentor, Harada and Nakamura 28 ), which is in contrast to the higher reported responses observed in postmenopausal women compared with men of a similar age( Reference Burdge, Powell and Calder 29 ). In response to a single oral vitamin A fat loading test, van Beek et al. ( Reference van Beek, de Ruijter-Heijstek and Erkelens 21 ) investigated whether a natural menopause was associated with reduced protection from exaggerated postprandial lipaemia. Higher concentrations of postprandial plasma TAG and retinyl palmitate (an indirect marker of CM) were observed in postmenopausal compared with premenopausal women of similar age, BMI, daily energy and fat intake, APOE genotype, LPL activity, and HDL-C concentration, even after adjusting for the confounding effect of fasting TAG. Relative to premenopausal women, Masding et al. ( Reference Masding, Stears and Burdge 23 ), Schoppen et al. ( Reference Schoppen, Perez-Granados and Navas-Carretero 22 ) and Jackson et al. ( Reference Jackson, Abraham and Smith 24 ) also reported significantly higher postprandial TAG responses after single and sequential fat-rich test meals in healthy postmenopausal women. Although raised LDL-cholesterol (LDL-C) is an established risk factor for CVD, large prospective studies have shown non-fasting TAG to be a better predictor of CVD risk in women than fasting LDL-C( Reference Bass, Newschaffer and Klag 30 – Reference Nordestgaard, Benn and Schnohr 32 ). Post hoc analysis of the Dietary Studies: Reading Unilever Postprandial Trials (DISRUPT) menopausal groups according to age also revealed a greater increase in non-fasting TAG than fasting LDL-C concentrations during the late premenopausal period, suggesting that age and the menopause have a differential impact on these two lipid CVD risk biomarkers( Reference Jackson, Abraham and Smith 24 ).
A major biochemical change that occurs in women after the menopause is a reduction in the secretion of endogenous oestrogen and progesterone( Reference Burger, Dudley and Robertson 33 ). These hormones not only play a major role in sexual physiology, but are also involved in various physiological processes associated with the vasculature and lipid metabolism. A reduction in oestrogen following the menopause is associated with a detrimental impact on lipoprotein metabolism, vascular reactivity and blood pressure (Fig. 1). For example, there is much evidence to suggest that oestrogen (endogenous and exogenous) lowers fasting plasma concentrations of total and LDL-C, lipoprotein(a) and apo B, whilst elevating HDL-C, apo AI and apo AII( Reference Žegura and Gužič-Salobir 34 – Reference Windler, Kovanen and Chao 36 ). The effects of oestradiol (the predominant type of oestrogen) on lipid metabolism is reported to contribute 25 % of its protective effects on the fasting lipid profile( Reference Gordon, Probstfield and Garrison 37 ). One possible mechanism to explain this effect, that was identified in in vitro animal studies, was an increase in the number of high-affinity LDL-receptors on liver cell membranes that enhance LDL uptake by the liver( Reference Windler, Kovanen and Chao 36 ). Furthermore, the administration of even short-term (2–6 weeks) oestradiol therapy reduces the menopause-related rise in postprandial TAG in postmenopausal women( Reference Bessesen, Cox‐York and Hernandez 38 , Reference Westerveld, Kock and Van Rijn 39 ). These findings indicate that 17β-oestradiol may accelerate the postprandial clearance of TRL and have a beneficial effect on postprandial lipaemia.
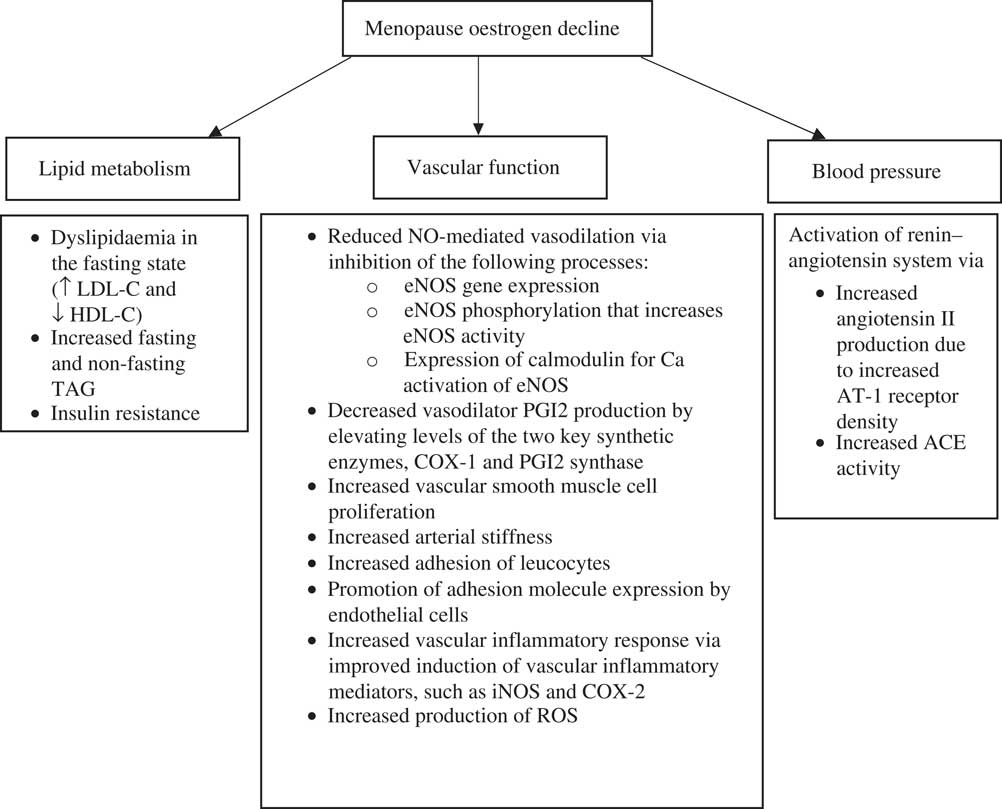
Fig. 1 Consequences of the decline in oestrogen during the menopause on lipid metabolism, vascular function and blood pressure. Adapted from Davis et al. ( Reference Davis, Lambrinoudaki and Lumsden 106 ). ACE, angiotensin converting enzyme; AT-1; angiotensin 1 receptor; COX, cyclo-oxygenase; eNOS, endothelial NO synthase; HDL-C, HDL-cholesterol; iNOS, inducible NO synthase; LDL-C, LDL-cholesterol; ROS, reactive oxygen species.
Vascular function and blood pressure
Vascular function is a measure of cardiovascular health. The components of impaired vascular function, including hypertension( Reference Huang, Wang and Cai 40 , Reference Ettehad, Emdin and Kiran 41 ), arterial stiffness( Reference Vlachopoulos, Aznaouridis and Stefanadis 42 ) and impaired endothelial dependent vasodilation (endothelial dysfunction)( Reference Ras, Streppel and Draijer 43 , Reference Matsuzawa, Kwon and Lennon 44 ), are all associated with cardiovascular mortality. In a healthy blood vessel, the endothelium, which is comprised of a monolayer of endothelial cells that lines the blood vessel walls, regulates vascular wall homeostasis by immediately responding to blood-borne and locally produced stimuli to regulate blood flow, blood pressure and vascular tone. It does so by maintaining a precise balance between the release of endothelium-derived vasodilators (such as NO) and vasoconstrictors (such as endothelin-I), which actively regulates vascular permeability to plasma constituents, platelets and leucocyte adhesion molecules( Reference Yao, Ober and Krishnaswami 45 ) as well as aggregation and thrombosis( Reference Cohen 46 ). However, when the synthesis or bioavailability of NO is reduced, the resulting imbalance of these vasoactive substances disrupts vascular homeostasis. This ‘endothelial dysfunction’ is characterised by vasoconstriction, increased expression of adhesion molecules and pro-inflammatory cytokines, platelet activation and increased oxidative stress( Reference Verma and Anderson 47 ), and is becoming increasingly recognised as an important step for the initiation of coronary atherosclerosis( Reference Widlansky, Gokce and Keaney 48 ) and increased CVD risk in postmenopausal women( Reference Rossi, Nuzzo and Origliani 49 ).
There are a number of non-invasive methods that are used to evaluate endothelial function( Reference Fichtlscherer, Rosenberger and Walter 50 ). Flow-mediated dilatation (FMD) is the ‘gold standard’ technique that uses ultrasound to assess endothelium-dependent vasodilation in the conduit arteries in the peripheral circulation and is used as a surrogate measure of NO production( Reference Moens, Goovaerts and Claeys 51 ). It is now recognised as a screening tool to assess future CVD risk( Reference Ras, Streppel and Draijer 43 , Reference Rossi, Nuzzo and Origliani 49 , Reference Inaba, Chen and Bergmann 52 , Reference Schächinger, Britten and Zeiher 53 ). Rossi et al. reported that postmenopausal women in the lowest tertile of percentage FMD response (reflective of impaired vascular reactivity) had the greatest relative risk of cardiovascular events. Furthermore, it has been shown that endothelial function is impaired across the stages of the menopause transition in healthy women with the highest percentage FMD response reported in premenopausal women, with a progressive decline in perimenopausal and postmenopausal women, respectively( Reference Moreau, Hildreth and Meditz 54 ). These findings suggest that the perimenopausal stage (the transition towards the menopause where oestrogen production starts to fall) is a crucial turning point in women where changes in CVD risk commence.
Majmudar et al. ( Reference Majmudar, Robson and Ford 55 ) revealed that menopausal status is associated with reduced NO activity, which is restored with oestrogen replacement therapy and may be an important mechanism facilitating the detrimental effect of the menopause on CVD risk and mortality. Another study that acutely administered oestrogen (17β-oestradiol) to postmenopausal women demonstrated protective effects on forearm microvascular responses to the endothelium-dependent vasodilator acetylcholine via improvements in NO activity( Reference Gilligan, Badar and Panza 56 ). Impaired blood flow in the microcirculation has been proposed to be an indicator of initial endothelial damage in individuals at risk of CVD( Reference Brodsky, Gealekman and Chen 57 ). Furthermore, it has been repeatedly shown that 17β-oestradiol stimulates the production of vasodilatory prostaglandins, such as prostacyclin (PGI2)( Reference Ospina, Duckles and Krause 58 , Reference Ospina, Krause and Duckles 59 ). These vascular effects are believed to be partly responsible for the long-term benefit of oestrogen therapy on cardiovascular risk in postmenopausal women. However, findings from the Women’s Health Initiative study have questioned the benefits of oestrogen therapy. After a short (6·8 years) or longer-term (18 years) follow-up relative to a placebo, oestrogen therapy did not protect against myocardial infarction or coronary death, although the findings did show a lower CHD risk among the younger postmenopausal women (50–59 years)( Reference Manson, Aragaki and Rossouw 60 , Reference Hsia, Langer and Manson 61 ). More recently, a systematic review involving 43 637 women reported the number of cardiovascular events to increase following the long-term (>1 year) use of oestrogen therapy( Reference Marjoribanks, Farquhar and Roberts 62 ), whereas a meta-analysis of peri- and post-menopausal women (n 40 048; aged 53–79 years) showed a risk reduction for fractures and diabetes but no significant impact on risk of CHD when oestrogen therapy was compared with a placebo( Reference Gartlehner, Patel and Feltner 63 ). In contrast, there is much evidence to suggest that oestrogens (endogenous and exogenous) have several cardioprotective effects (Fig. 1)( Reference Vehkavaara, Silveira and Hakala-Ala-Pietilä 35 , Reference Žegura, Gužic-Salobir and Šebeštjen 64 , Reference Knopp, Zhu and Bonet 65 ). These include reductions in plasma markers of endothelial activation (E-selectin), fibrinogen, plasminogen activator inhibitor type 1 and tissue plasminogen activator antigen( Reference Vehkavaara, Silveira and Hakala-Ala-Pietilä 35 , Reference Vigen, Hodis and Chandler 66 ). However, increases in plasma markers of inflammation (C-reactive protein) and hypercoagulability have also been reported( Reference Vehkavaara, Silveira and Hakala-Ala-Pietilä 35 , Reference Žegura, Gužic-Salobir and Šebeštjen 64 ).
Hypertension (high blood pressure) is one of the main age-related disorders in postmenopausal women( Reference Wassertheil-Smoller, Anderson and Psaty 67 , Reference Nash, Magder and Lustberg 68 ), which has been identified as a leading risk factor for myocardial infarction and stroke in women( Reference Abramson and Melvin 69 ). The renin–angiotensin system (RAS) is a hormonal cascade that plays a key role in the regulation of fluid and electrolyte balance and arterial blood pressure. Upon activation of the RAS cascade, angiotensin II is produced in the liver by angiotensin-converting enzyme (ACE) following conversion of angiotensin I to angiotensin II( Reference Donoghue, Hsieh and Baronas 70 ). Angiotensin II is a potent vasoconstrictor which degrades bradykinin (a vasodilator) causing arterioles to constrict, resulting in increased blood pressure( Reference Leung 71 ). It is well documented in the literature that oestrogen acts on RAS at different points of the cascade, including the inhibition of ACE activity. In vitro and in vivo animal studies have also demonstrated the potential effects of oestrogen on the endothelial-dependent vasodilator response to acetylcholine in coronary and uterine arteries( Reference Bell 72 – Reference Williams, Adams and Herrington 74 ). Loss of oestrogen-dependent cardiovascular protection induces endothelial dysfunction, and may also be involved in the activation of the RAS cascade. Evidence from both clinical and animal studies has shown an inverse association between oestrogen and the activation of RAS( Reference Hinojosa-Laborde, Craig and Zheng 75 – Reference Schunkert, Danser and Hense 78 ). This has been proposed to occur due to oestrogen-induced down-regulation of angiotensin receptor I expression leading to an augmented level of angiotensin II( Reference Nickenig, Bäumer and Grohè 76 ). This is a major component of the RAS system and has several harmful effects on the vascular wall including vasoconstriction, vascular smooth muscle cell proliferation, reactive oxygen species generation and endothelial cell apoptosis( Reference Ginnan, Guikema and Halligan 79 – Reference Wassmann, Wassmann and Nickenig 81 ). Oestrogen deficiency has also been reported to lead to an up-regulation of ACE activity causing an accumulation of angiotensin II( Reference Fischer, Baessler and Schunkert 82 ).
Impact of meal fat composition on postprandial lipaemia and vascular function
The chronic effects of substitution of SFA with PUFA on fasting lipid levels have been extensively studied( Reference Mozaffarian, Micha and Wallace 83 ), however, the acute effects are less well known. One systematic review and meta-analysis of randomised controlled trials (RCT) compared the effects of oral fat tolerance tests with differing fatty acid compositions on postprandial TAG responses in men and women( Reference Monfort-Pires, Delgado-Lista and Gomez-Delgado 84 ). Relative to a single SFA-rich meal challenge, a PUFA-rich meal significantly reduced the postprandial lipaemic response over 8 h, whereas a trend for a reduced response was identified following a MUFA-rich meal. However, differences were not evident at 4 h, suggesting that a longer follow-up time after the test meal (i.e. 8 h) is preferable to observe the acute effects of meal fat composition on postprandial lipaemia. Of the eighteen studies included in the review by Monfort-Pires et al.( Reference Monfort-Pires, Delgado-Lista and Gomez-Delgado 84 ), none included postmenopausal women, which reflects the paucity of postprandial data in this population subgroup.
With regards to vascular function, West( Reference West 85 ) suggested that consumption of a single high-fat meal (50–105 g of fat) can impair postprandial FMD by 45 to 80 %, which appears to occur within 2 to 5 h after a high-fat meal( Reference Ong, Dean and Hayward 86 – Reference Vogel, Corretti and Plotnick 89 ). Prolonged postprandial lipaemia is known to induce endothelial dysfunction by promoting the formation of free radicals by accelerating the rate of β-oxidation of NEFA (for example, superoxide radicals). Increased production of reactive oxygen species or free radicals reduces the amount of bioactive NO by chemical inactivation to form toxic peroxynitrite( Reference Pacher, Beckman and Liaudet 90 ). In addition, it has been shown that persisting oxidative stress will render endothelial NO synthase dysfunctional, markedly reducing NO production( Reference Förstermann 91 ). Furthermore, high concentrations of TRL during the postprandial state enhance inflammation by inducing the secretion of pro-inflammatory cytokines( Reference Margioris 92 ) and expression of soluble cell adhesion molecules( Reference Rubin, Claas and Pfeuffer 93 ).
Reviews by Hall( Reference Hall 94 ) and Vafeiadou et al. ( Reference Vafeiadou, Weech and Sharma 95 ) stated that the acute effects of dietary fats on vascular function are less researched. The authors concluded that high-fat meals have a detrimental effect on postprandial vascular function and that there is limited and inconclusive evidence for the comparative effects of test meals rich in MUFA or n-6 PUFA with SFA. Of note, the data derived from these reviews were mainly from studies where the effects of a single high-fat meal on postprandial vascular function in different subject groups were determined; however, none of the studies identified included postmenopausal women only.
Methods
A systematic approach was used to identify all relevant published literature according to the method used by Vafeiadou et al. ( Reference Vafeiadou, Weech and Sharma 95 ). The PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) database was used to perform the literature search, which included all studies published in English until October 2016. A protocol that included search terms to conduct the literature search was prepared by two authors (K. M. R. and M. W.), which was agreed by all authors. Three categories of search terms were identified: (i) study group search term (postmenopausal or postmenopausal or post-menopause or menopause or menopausal); (ii) exposure search terms (which included descriptors of SFA, MUFA and n-6 PUFA, and relevant food sources, for example, butter, safflower oil and olive oil); and (iii) outcomes (which included descriptors of vascular function, blood pressure, biomarkers of vascular function and inflammation, and plasma lipids) (Supplementary information). The Medical Subject Heading Browser (http://www.nlm.nih.gov/mesh/MBrowser.html) was used to identify relevant exposures and outcomes. Two additional studies were identified through hand searching of original articles found using the PubMed search. The titles and abstracts of every paper identified from the search were assessed for relevance by one author (K. M. R.) and any uncertainties were discussed with other members of the review team until a consensus was reached. This review was restricted to epidemiological studies (cross-sectional, case–control and cohort) and RCT in postmenopausal women with respect to test meals rich in SFA, MUFA and/or n-6 PUFA. Only published peer-reviewed literature was considered (i.e. ‘grey’ literature, such as dissertations, conference proceedings, reports, letters to editors and other non-peer-reviewed research, was excluded). In the present review, we only considered acute studies as our objectives were to determine the impact of meal fatty acids on non-fasting TAG responses, vascular function, blood pressure, and biomarkers of vascular function and inflammation as important CVD risk factors in postmenopausal women. Fig. 2 presents a summary of the literature search and reasons for exclusion of the studies.
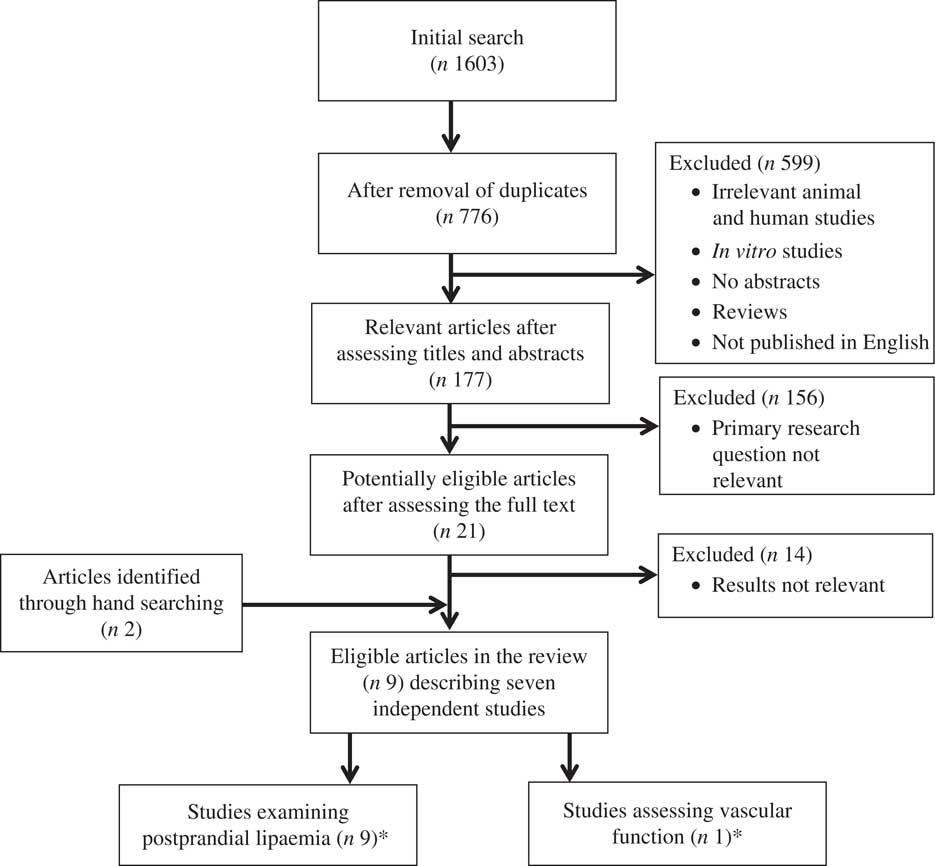
Fig. 2 Flow of information through the different phases of the review. * Of the studies included, one publication reported data on both postprandial lipaemia and vascular function.
Results and discussion
This systematic search identified 778 publications in total. Of these, there were nine relevant articles describing seven independent studies in postmenopausal women that examined the acute effects of meals enriched in SFA and/or MUFA and/or n-6 PUFA on postprandial lipaemia( Reference Alssema, Schindhelm and Dekker 96 – Reference Westerveld, Meijer and Erkelens 104 ). One of these studies also determined the impact of a single high-fat meal with a PUFA:SFA ratio of 0·06 on vascular reactivity( Reference Siepi, Marchesi and Lupattelli 101 ) (Table 1). No studies were identified that reported the acute effects of meal fatty acids on postprandial blood pressure, or biomarkers of vascular function and inflammation in postmenopausal women. Only one single-blind RCT compared the effects of meal fat composition on postprandial lipaemia using a sequential meal protocol, the results of which were presented in three publications( Reference Jackson, Robertson and Fielding 97 , Reference Jackson, Robertson and Fielding 98 , Reference Robertson, Jackson and Fielding 100 ). As opposed to a single-meal protocol, the use of a multiple-meal design by the researchers is considered superior because it more closely mimics the eating pattern of free-living individuals, particularly in Westernised societies, and provokes a sustained lipaemic response. Five publications described cross-sectional epidemiological studies, which were single-arm studies that did not include comparator meals, and whose fatty acid compositions varied( Reference Alssema, Schindhelm and Dekker 96 , Reference Siepi, Marchesi and Lupattelli 101 – Reference Westerveld, Meijer and Erkelens 104 ). Among these postprandial studies with blood samples collected between 6 to 10 h after the test meal, two studies( Reference Alssema, Schindhelm and Dekker 96 , Reference Silva, Wright and Williams 102 ) used a sequential two-meal protocol, whereas the other three studies( Reference Siepi, Marchesi and Lupattelli 101 , Reference Wassef, Salem and Bissonnette 103 , Reference Westerveld, Meijer and Erkelens 104 ) incorporated a single-meal approach. In addition, one case–control study was identified that considered the responses of normolipaemic, hypercholesterolaemic and mixed hyperlipidaemic postmenopausal women to a single high-fat meal( Reference Pirro, Lupattelli and Siepi 99 ).
Table 1 Acute test-meal studies investigating the effects of meal fat content and composition on postprandial lipaemia and vascular function in postmenopausal women

HDL-C, HDL-cholesterol; ↓, decrease over time relative to baseline (fasting) unless otherwise specified; ↑, increase over time relative to baseline (fasting) unless otherwise specified; TC, total cholesterol; Lp(a), lipoprotein(a); PoM, postmenopausal women; %E, energy percentage; CHO, carbohydrate; CETP, cholesteryl ester transfer protein; IAUC, incremental AUC; FMD, flow-mediated dilatation; GSH, glutathione.
* No comparator group.
† Fatty acid values given per 100 g of test oil, of which 41 g was included in the breakfast.
Data on these human studies will be presented in two sections that address the effects of fatty acid composition on (i) postprandial lipaemia and (ii) postprandial vascular function in postmenopausal women.
Acute effects of meal fat composition on postprandial lipaemia
The five cross-sectional studies, investigating both single and sequential meals, provide consistent evidence that fat-rich loads, irrespective of fatty acid composition, augment postprandial TAG in postmenopausal women( Reference Alssema, Schindhelm and Dekker 96 , Reference Siepi, Marchesi and Lupattelli 101 – Reference Westerveld, Meijer and Erkelens 104 ) (Table 1). Furthermore, Pirro et al. ( Reference Pirro, Lupattelli and Siepi 99 ) reported a significantly greater postprandial TAG response at 4, 6 and 8 h after a standardised oral fat load (65 g of fat) in mixed hyperlipidaemic women compared with hypercholesterolaemic and normolipidaemic women, which may reflect their higher baseline TAG concentrations. As expected, other factors involved in lipid metabolism, including increases in apo B-48( Reference Silva, Wright and Williams 102 ), glucose( Reference Wassef, Salem and Bissonnette 103 ) and insulin( Reference Wassef, Salem and Bissonnette 103 ) as well as reductions in HDL-C( Reference Pirro, Lupattelli and Siepi 99 , Reference Wassef, Salem and Bissonnette 103 , Reference Westerveld, Meijer and Erkelens 104 ), glutathione( Reference Siepi, Marchesi and Lupattelli 101 ) and NEFA( Reference Wassef, Salem and Bissonnette 103 ) were also observed postprandially compared with fasting values. However, comparison of the findings from the different studies are challenging due to differences in the nature of the fats and oils used in the test meal, the amount and composition of fat, and postprandial follow-up times, as well as the use of both single and sequential test meal protocols. They are also limited in their cross-sectional design in that the lack of comparator meals prevents any conclusions from being made regarding the impact of meal fat composition on postprandial lipaemia. Among all nine articles (seven independent studies) reported in Table 1, only one study described in three publications compared the postprandial lipaemic responses to test meals containing oils rich in SFA (palm oil), MUFA (olive oil), n-6 PUFA (safflower oil) and a mixture of n-6 PUFA and n-3 PUFA (safflower and fish oils)( Reference Jackson, Robertson and Fielding 97 , Reference Jackson, Robertson and Fielding 98 , Reference Robertson, Jackson and Fielding 100 ). In this study by Jackson and colleagues( Reference Jackson, Robertson and Fielding 97 , Reference Jackson, Robertson and Fielding 98 , Reference Robertson, Jackson and Fielding 100 ), ten postmenopausal women ingested a high-fat breakfast containing 40 g of the assigned test fat followed by a low-fat, high-carbohydrate lunch (5·4 g total fat) given 5 h later. The authors observed significantly higher concentrations of plasma NEFA and lower insulin sensitivity following the SFA meal compared with the other test oils. During the postprandial state it has been shown that up to 50 % of the liberated NEFA is dietary-derived CM-TAG due to the action of LPL upon TAG to release NEFA( Reference Robertson, Jackson and Fielding 100 ). Although Robertson et al. ( Reference Robertson, Jackson and Fielding 100 ) did not determine the specific fatty acid composition of the circulating NEFA after consumption of the meals, a similar study reported the postprandial change in the plasma NEFA profile to represent the fatty acid composition of the test meals( Reference Fielding, Callow and Owen 105 ). Based on the same sequential-meal study, Jackson et al. ( Reference Jackson, Robertson and Fielding 98 ) further examined the postprandial TAG and apo B-48 (the apolipoprotein specifically associated with CM) responses, including the responses in three distinct TRL subfractions, and reported significant differences in the apo B-48 time-course profiles between the four different test oils( Reference Jackson, Robertson and Fielding 98 ). In particular, the MUFA meal resulted in the formation of a greater number of both large (Svedberg flotation rate (Sf) >400 fraction) and moderately (Sf 60–400 fraction) sized apo B-48 particles compared with the other three study meals. The findings from this study suggested that olive oil may enhance CM formation and Jackson et al. ( Reference Jackson, Robertson and Fielding 97 ) hypothesised that MUFA may modify the activity or expression of intestinal microsomal TAG transfer protein, which is involved with TRL assembly.
Acute effects of meal fat composition on vascular function
Only one study examined the acute impact of total fat and/or SFA and/or MUFA and/or n-6 PUFA on vascular reactivity in postmenopausal women. A significant decrease in the percentage FMD response at 2 h (2·3 (sd 2·6) %) compared with baseline (7·7 (sd 2·8) %; P<0·05) was observed in healthy women after a 65 g oral fat load with a PUFA:SFA ratio of 0·06( Reference Siepi, Marchesi and Lupattelli 101 ) (Table 1). The lack of comparator meal in this study makes it difficult to draw conclusions regarding the impact of fatty acid composition on vascular function in postmenopausal women.
Summary
A systematic approach was used to review the literature on the impact of meal fat composition (SFA, MUFA and n-6 PUFA) on postprandial lipaemia, blood pressure, vascular function and biomarkers of vascular function and inflammation in postmenopausal women. However, there is at present an extremely limited number of RCT that have investigated the effects of meal fatty acid composition on measures of postprandial lipaemia and vascular function in this population subgroup. Furthermore, differences in study designs (such as the absence of a comparator test meal, and differences in meal fat composition, study duration and outcome measures) prevent any firm conclusions being drawn from the present literature review.
Conclusions
In conclusion, there is an urgent requirement for suitably powered RCT to investigate the effects of meal fat composition on postprandial lipaemia and vascular function in postmenopausal women. With the increased prevalence of non-communicable diseases in women, especially after the menopause, future studies should consider both healthy postmenopausal women and those at increased cardiometabolic risk using well-standardised measures of vascular function. Since non-fasting TAG is an important CVD risk factor for women, it would be preferable to use robust test-meal protocols that are more reflective of habitual eating patterns to gain a greater understanding of the day-long postprandial handling of different dietary fats.
Acknowledgements
K. M. R. was supported by the Commonwealth Scholarship Commission, UK. This research received no specific grant from any funding agency, commercial or non-profit sectors.
The authors’ responsibilities were as follows: K. M. R., M. W., K. G. J. and J. A. L. contributed to the conception of the literature search strategy. K. M. R. undertook the literature search, extracted and interpreted the data from the literature and wrote the manuscript. M. W., K. G. J. and J. A. L. critically appraised the document at all stages. J. A. L. was responsible for the final content.
None of the authors has any conflicts of interest.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0954422418000033





