Introduction
Since ancient times, many herbal medicines in different formulations have been recommended for the treatment of various diseases. Traditional and/or indigenous drugs have special significance of having been tested over a long time, and are relatively safe, easily available and affordable. Many ethno-botanical surveys on medicinal plants used by the local population have been performed in different parts of the world including the USA, China, India, Mexico, Morocco, Saudi Arabia, Taiwan, and Trinidad and Tobago(Reference Atta-Ur-Rahman1–Reference Mahabir and Gulliford4), and suggested that several medicinal plants have been used as dietary adjuncts for the treatment of numerous chronic and severe diseases. In India and China, the use of herbal medicines has been commonly practised for a long time as a less expensive way to treat various health problems. The herbal drugs are considered frequently less toxic with limited side effects compared with synthetic drugs(Reference Geetha, Biju and Augusti5, Reference Rao, Sudarshan and Rajsekher6). For such reasons, traditional and complementary medicines have seen an upsurge in their popularity for the treatment of different diseases. Herbal medicine development is one of the main subjects of studies in the National Center for Complementary and Alternative Medicines, Bethesda, USA which was established in 1998 by the US Government(Reference Edwards, Colquist and Maradiegue7, Reference Fan8). The WHO has also recommended the initiation of studies to identify and characterise new herbal preparations from traditionally known plants and the development of new effective therapeutic agents, especially in the areas where we lack safe modern drugs to treat chronic diseases(9, Reference Okerele10). In the ongoing search for more effective and safer drugs, attention is being paid to new and safe medicinal herbs or food components(Reference Edwards, Colquist and Maradiegue7, Reference Okerele10). Although phyto-therapy continues to be used in several countries as in the past, only a few plants have received scientific or medical scrutiny. Although most of the medicinal plants are safer, still a number of medicinal plants possess some degree of toxicity; therefore it is very important to analyse the traditional therapeutic regimens scientifically and validate their dosing, toxicity and other health consequences, before proper use in human diseased conditions. In the present article we discuss the biological and medicinal potential of a well-known edible plant, pumpkin (genus Cucurbita; family Cucurbitaceae). Pumpkin has various health benefits, which are summarised in Fig. 1.
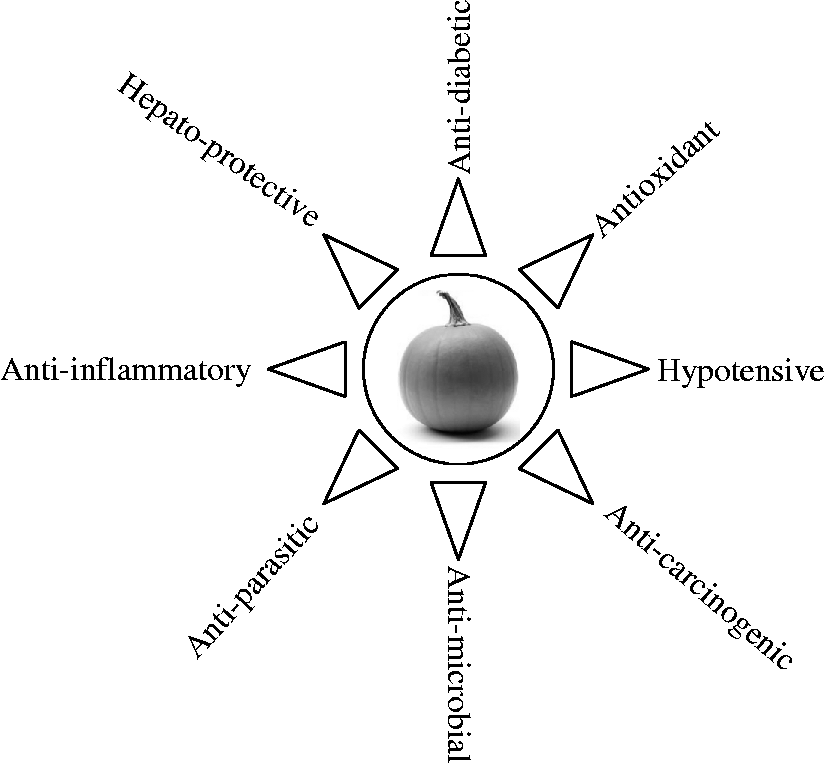
Fig. 1 Medicinal properties of pumpkins.
Pumpkin used as a good edible plant
Pumpkin is cultivated from northern Mexico to Argentina and Chile and has spread to Europe (France and Portugal, for example), Asia (India and China) and Western America. Pumpkin is an annual vine or trailing plant and can be cultivated from sea level to high altitudes. It is famous for its edible seeds, fruit and greens(Reference Stovel11). The most important part of pumpkin is its low-fat and protein-rich seeds(Reference Matsui, Guth and Grosch12). The second most important part is its fruit. The immature fruit is cooked as a vegetable, while the mature fruit is sweet and used to make confectionery and beverages, sometimes alcoholic. The fruit has a good β-carotene content and has a moderate content of carbohydrates, vitamins and minerals (Table 1). Different parts of the pumpkin plant have been used in the form of various food regimens throughout its distribution area in America. The unripe fruit is eaten as a boiled vegetable, while the flesh of the ripe fruit is used to prepare sweets and soft or slightly alcoholic drinks. Seeds are also greatly valued and in Chiapas, Mexico, they are used with honey to prepare desserts known as palanquetas. Edible oil is also obtained from the seed of pumpkin which is rich in oleic acid. Many varieties of pumpkins are available, and some of them are described elsewhere(Reference Robinson and Decker-Walters13).
Table 1 Nutrients in pumpkin*
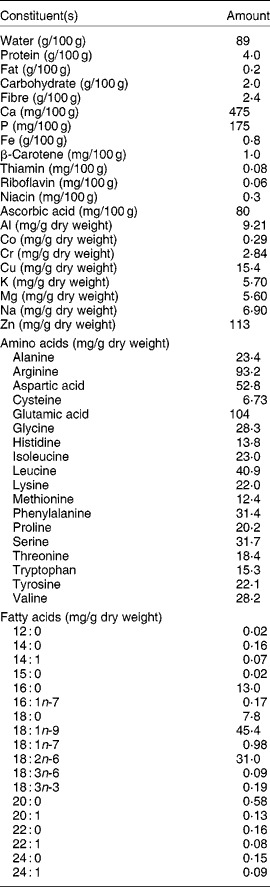
* The sources of the data in the table were USDA Nutrient Composition Tables, various studies including de Escalada Pla et al. (65) and other websites.
Botanical description
Pumpkin is a creeping or climbing plant, monoecious, annual although persistent for a certain period, giving the impression of being a short-lived perennial, without swollen reserve roots. It is resistant to low temperatures but not to severe frosts. It has five vigorous, slightly angular stems and leaves with 5 to 25 cm petioles that are ovate–cordate to suborbicular–cordate, with or without white spots on the surface and have three to five rounded or obtuse, apiculate lobules, the central one bigger than lateral ones. Male flowers are long and pedicellate and have a campanulate calyx that is 5 to 10 mm long and almost as wide, 5–15 × 1–2 mm linear sepals and a tubular campanulate corolla that is rather broader towards the base, 6 to 12 cm long and yellow to pale orange. They have three stamens. Female flowers have sturdy peduncles, 3 to 5 cm long, an ovoid to elliptical, multilocular ovary, sepals that are occasionally foliaceous and a corolla that is somewhat larger than that of the male flowers. They have a thickened style and three lobate stigmas. The fruit is globose to ovoid–elliptical, with three colour patterns: (1) light or dark green, with or without longitudinal white lines or stripes towards the apex; (2) minutely spotted white and green; (3) orange, white, cream or flesh white. The flesh is sweet and the seeds are ovate–elliptical, flattened, 15–25 × 7–12 mm, and a dark brown to black or creamy white colour(Reference Whitaker and Davis14).
Phytochemistry of pumpkin
Pumpkin has been considered as beneficial to health because it contains various biologically active components such as polysaccharides, para-aminobenzoic acid, fixed oils, sterols, proteins and peptides(Reference Caili, Huan and Quanhong15–Reference Murkovic, Mulleder and Neunteufl17). The fruits are a good source of carotenoids and γ-aminobutyric acid(Reference Murkovic, Mulleder and Neunteufl17, Reference Matus, Molnár and Szabó18). Pumpkin seeds (Cucurbita spp.) are valued for their high protein content(Reference Mansour, Dworschak and Pollhamer19) and useful amounts of the essential fatty acid, linoleic acid(Reference Glew, Glew and Chuang20). Pumpkin seeds contain remarkably high proportions of essential amino acids(Reference Glew, Glew and Chuang20). Pumpkin seeds also contain relatively large amount of various essential micro-elements such as K, Cr and Na (Table 1). Pumpkin seeds are a good source of Mg, Zn, Cu, Mo and Se, etc. From pumpkin leaves and germinated seeds, several phytochemicals such as polysaccharides, phenolic glycosides, NEFA and proteins have been isolated(Reference Nwokolo and Sim21, Reference Koike, Li and Liu22). Various hypoglycaemic polysaccharides have been characterised from fruit pulps of pumpkin plants(Reference Jun, Lee and Song23). d-chiro-Inositol in pumpkin has been identified as an insulin secretor and sensitiser(Reference Xiong24). Various antibiotic components including anti-fungal components have been characterised from various parts of pumpkin plants. Various anti-fungal proteins, such as α- and β-moschins (molecular weight (MW) 12 kDa), myeloid antimicrobial peptide (MAP)-28 (MW 28 kDa), MAP2 (MW 2·2 kDa), MAP4 (MW 4·6 kDa), MAP11 (MW 11·6 kDa) and a peptide (MW 8 kDa) from pumpkin have been isolated and characterised(Reference Vassiliou, Neumann and Condron25). The structures of some of these components are represented in Fig. 2.
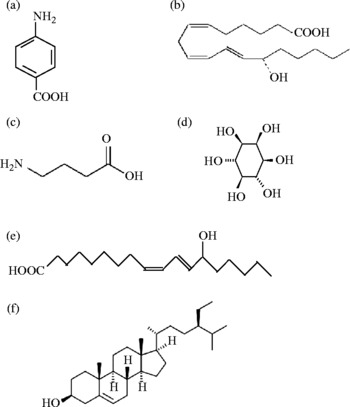
Fig. 2 Structures of some compounds isolated from pumpkins: (a) para-aminobenzoic acid; (b) 11E-octadecatrienoic acid; (c) γ-aminobutyric acid; (d) d-chiro-inositol; (e) 13-hydroxy-9Z; (f) β-sitosterol.
Medicinal bioactivities of pumpkin
Although pumpkin is a well-known edible plant, most parts of this plant are also used in traditional systems of medicine around the world. Although a large number of compounds have been isolated from pumpkin spp.(Reference Caili, Huan and Quanhong15), only some of them have biological activities and medicinal properties, which are described in the following sections. Table 2 summarises the bioactive compounds from pumpkin and their medicinal properties.
Table 2 Important bioactive compounds from pumpkin and their biological activities

TBARS, thiobarbituric acid-reactive substances; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase; MAP, myeloid antimicrobial peptide; MW, molecular weight.
Anti-diabetic activity
With the rapidly increasing prevalence of diabetes and its high economic burden in the world population, the scientific community has been called upon to develop new safer and inexpensive medicines for the treatment of diabetes. Herbal medicines fulfil these requirements. Therefore, various studies have been recently conducted to recognise the anti-diabetic potential of herbal formulations; pumpkin is one of them, which is a normally cultivated plant in farms and its fruits are used for human consumption in diabetic conditions(Reference Xia and Wang26, Reference Kwon, Apostolidis and Kim27). Local healers recommend the ingestion of crude aqueous extract of pumpkin fruits for the treatment of type 2 diabetes or non-insulin-dependent diabetes mellitus(Reference Kwon, Apostolidis and Kim27, Reference Acosta-Patiño, Jiménez-Balderas and Juárez-Oropeza28). In various other reports, the pumpkin exhibited acute hypoglycaemic activity (blood sugar lowering) in temporarily hyperglycaemic rabbits, in alloxan-induced diabetic rabbits, and in type 2 diabetic patients(Reference Acosta-Patiño, Jiménez-Balderas and Juárez-Oropeza28–Reference Alarcon-Aguilar, Hernandez-Galicia and Campos-Sepulveda30). Xia & Wang(Reference Xia and Wang31) demonstrated that pumpkin has hypoglycaemic activity like a standard drug (tolbutamide) in healthy animals with temporary hyperglycaemia and in mild diabetic animals, but not in severe diabetic animals. They suggested that these effects might be due to either increased pancreatic insulin secretion from the existing β-cells or insulin release from the bound form. d-chiro-Inositol was identified in pumpkin (especially in Cucurbita ficifolia) and this compound has been considered as an insulin action mediator (insulin sensitiser)(Reference Xia and Wang32). However, the detailed mechanism of anti-diabetic action of this component remains to be clarified.
Various other components have also been isolated from pumpkin and analysed for anti-diabetic potential. For example, Kwon et al. (Reference Kwon, Apostolidis and Kim27) reported that phenolic phytochemicals of pumpkin have anti-diabetic effects in terms of β-glucosidase and α-amylase inhibition. Pumpkin also has hypotensive effects in terms of angiotensin I-converting enzyme-inhibitory activities. Furthermore, Quanhong et al. (Reference Quanhong, Caili and Yukui33) also investigated hypoglycaemic substances from pumpkin, and they isolated protein-bound polysaccharide by activity-guided isolation from water-soluble substances of the pumpkin fruits. When this protein-bound polysaccharide from pumpkin fruits (PBPP) was evaluated for hypoglycaemic activity and effects on serum insulin levels in alloxan diabetic rats, and it was found that PBPP can increase the levels of serum insulin, reduce the blood glucose levels and improve tolerance of glucose in alloxan-induced diabetic animals. By considering all these facts, it can be concluded that pumpkin has potential anti-diabetic properties, which may suggest the inclusion of this plant in anti-diabetic regimens to treat human diabetes. However, further studies in detail are warranted to explore the mechanistic and therapeutic potential of pumpkins for diabetes.
Antioxidant activity
Oxidative stress has been considered as a hallmark of various chronic diseases and their complications such as diabetes, obesity, CVD and cancer. It is a condition of potentially harmful imbalance between the level of pro-oxidants and antioxidants in favour of the former(Reference Halliwell34). Various extracts of pumpkin have potential antioxidant activity which might play an important role in pre-diabetics, diabetics and individuals with vascular injury. Xia & Wang(Reference Xia and Wang31) demonstrated the hypoglycaemic action of pumpkin (fruit) extract as well as its role as an antioxidant to reveal a mechanism for its cytoprotective (cell-protecting) action in streptozotocin-induced diabetic animals. Pumpkin seeds have a high content of vitamin E (tocopherol; an antioxidant), and pumpkin seed oil has been considered to provide a significant source of vitamin E in Japanese diets(Reference Imaeda, Tokudome and Ikeda35). Dang(Reference Dang36) reported that pumpkin extract administration significantly increased the serous and hepatic activities of superoxide dismutase and glutathione peroxidase in mice, and reduced the concentration of malonaldehyde. It has also been found that pumpkin polysaccharide could increase the superoxide dismutase and glutathione peroxidase activity and reduce the malonaldehyde content in tumour-containing mice serum(Reference Xu37).
Anti-carcinogenic effect
Cancer is a rapidly growing health problem; it presents the biggest challenge to researchers and medical professionals and has been selected for various prevention and therapeutic strategies. The dietary intake of many vegetables and fruits has been found to reduce the risk of occurrence of cancer(Reference Craig38). Diets high in pumpkin seeds have also been associated with lower risk of gastric, breast, lung and colorectal cancers(Reference Huang, Hirose and Wakai39). There are also potential health benefits, including anti-carcinogenic effects, to be gained from the various carotenoid pigments found in pumpkin seed oil(Reference Jian, Du and Lee40). The carotenoids from pumpkin fruits have been linked to the prevention of prostate cancer(Reference Jian, Du and Lee40, Reference Binns, Jian and Lee41). There are still various controversies regarding the use of juices of pumpkin fruits in cancer situations; for example, boiled pumpkin juice significantly suppressed the incidence of aberrant cells while fresh pumpkin juice enhanced it(Reference Hong42). It was reported that pumpkin fruit extracts markedly reduced tumour weight in S-180-bearing mice(Reference Hong42). Cheong et al. (Reference Cheong, Choi and Kim43) isolated some basic proteins from pumpkin seeds named MAP2 (MW 2249 Da) and MAP4 (MW 4650 Da), and reported inhibition of the growth of leukemia K-562 cells. Moreover, other proteins from pumpkin seeds were reported to inhibit melanoma proliferation(Reference Xie44). Xia et al. (Reference Xia, Li and Li45) isolated a novel ribosome-inactivating protein (RIP) called moschatin from the mature seeds of pumpkin (C. moschata) and a novel immunotoxin moschatin-Ng76 was prepared successfully which efficiently inhibits the growth of targeted melanoma cells M21 with an IC50 (50 % inhibitory concentration) of 0·04 nm, 1500 times lower than that of free moschatin. Recently, Hou et al. (Reference Hou, Meehan and Xie46) isolated a novel type 1 RIP designated cucurmosin from the sarcocarp of C. moschata that exhibits strong cytotoxicity to three cancer cell lines of both human and murine origin, besides rRNA N-glycosidase activity.
Antimicrobial activity
Diseases caused by bacteria, viruses, fungi and other parasites are major causes of death, disability, and social and economic disruption for millions of individuals. Despite the existence of safe and effective interventions, many individuals lack access to needed preventive and treatment care. Increasing drug resistance in infectious micro-organisms has warranted the development of new drugs against pathogenic micro-organisms. In this regard, natural sources have been considered as the best option to isolate new and novel anti-microbial components. Various broad-spectrum anti-microbial components have been isolated from pumpkins. Pumpkin oil inhibits Acinetobacter baumanii, Aeromonas veronii biogroup sobria, Candida albicans, Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Salmonella enterica subsp. enterica serotype typhimurium, Serratia marcescens and Staphylococcus aureus at the concentration of 2·0 % (v/v)(Reference Hammer, Carson and Riley47). A peptide (MW 8 kDa) from pumpkin seeds was proved to inhibit Botrytis cinerea, Fusarium oxysporum and Mycosphaerella arachidicola at a dose of 375 μg and to exert an inhibitory effect on cell-free translation with an IC50 (50 % inhibitory concentration) of 1·2 μm(Reference Vassiliou, Neumann and Condron25). Purified α-moschin and β-moschin, two proteins with a MW of 12 kDa from fresh brown pumpkin seeds, displayed translation-inhibiting activity with IC50 of 17 μm and 300 nm, respectively(Reference Xiong24). A significant inhibitory effect of a purified protein (MW 28 kDa) against the fungal growth of Fusarium oxysporum was exerted in an agar disc plate at a concentration greater than 2 mm. This protein possessed a synergistic effect with nikkomycin, a chitin synthase inhibitor, for the growth inhibition of Candida albicans (Reference Ng, Parkash and Tso48). Three pumpkin seed basic proteins, MAP2 (MW 2·2 kDa), MAP4 (MW 4·6 kDa) and MAP11 (MW 11·7 kDa), have been shown to inhibit the growth of yeast cells, with MAP11 being the most effective inhibitor. However, MAP2 and MAP4 did not inhibit the growth of the Gram-negative bacterium E. coli (Reference Cheong, Choi and Kim43). Moreover, it has been reported that phloem exudates from pumpkin fruits possess anti-fungal activities via inhibition of pathogenic fungal proteases(Reference MacGibbon and Mann49). Recently, Park et al. (Reference Park, Lee and Kim50) isolated a new protein called Pr-1 from pumpkins which has potential anti-fungal activity, without toxicity for human erythrocytes. It is a thermostable protein that is stable up to 70°C, without showing growth-arresting activity towards E. coli or Staphylococcus aureus (Reference Park, Lee and Kim50). By considering these facts, it is of great importance that those living in developing countries be encouraged to consume pumpkin, as it protects against organisms that cause infectious diseases in these regions of the world.
Other medicinal effects
Pumpkin-supplemented foods are considered as a good source of anti-inflammatory substances, which can help in many diseases such as arthritis, etc. Fahim et al. (Reference Fahim, Abd-el Fattah and Agha51) reported that pumpkin seed oil significantly inhibited adjuvant-induced arthritis in rats, similar to a well-known anti-inflammatory substance called indomethacin. It may well be considered that the supplementation of natural components with standard drugs might give synergistic, antagonistic and no-change effects (called drug interaction effects) during treatment of diseased conditions. Similarly, Fahim et al. (Reference Fahim, Abd-el Fattah and Agha51) tested the drug interaction effects of pumpkin seed oil with indomethacin and they found no effect in the adjuvant-induced arthritis model in rats. Pumpkin seed oil has potential hypotensive activity, as suggested by Zuhair et al. (Reference Zuhair, Abd El-Fattah and El-Sayed52). They also suggested that pumpkin seed oil has a very good drug interaction with hypotensive drugs such as felodipine (Ca antagonist) and captopril (an angiotensin-converting enzyme inhibitor), in regards to enhanced hypotensive potential in hypertensive animal models. Supplementation of pumpkin seed snacks showed a higher level of inhibitor of crystal formation or aggregation which will subsequently reduce the risk of bladder stone disease in the Thailand population(Reference Suphiphat, Morjaroen and Pukboonme53). Pumpkin seeds or orthophosphate supplementation at 60 mg/kg (body weight) per d could reduce the incidence of bladder stones; the longer the supplementation period of pumpkin seeds, the better the results that can be found(Reference Suphakarn, Yarnnon and Ngunboonsri54). It was reported that the oil preparation could remarkably reduce bladder pressure, increase bladder compliance and reduce urethral pressure. Shishigatani pumpkin possessed bio-antimutagenicity from the chloroform and ethyl acetate fractions(Reference Nakamura, Suganuma and Kuyama55). Pumpkin may ease depression too, because the seeds contain l-tryptophan, which raises levels of ‘happy’ serotonin in the brain(Reference Eagles56). The effect of water extracts of pumpkin seeds in the treatment of puppies experimentally infected with heterophyiasis gave promising results, and the combined extracts of areca nut and pumpkin seeds gave a better result than when either extract was given alone(Reference Mahmoud, Basiouny and Dawoud57). An anti-helminthic effect was reported at the minimum inhibitory concentration of 23 g pumpkin seed in 100 ml distilled water in preclinical studies(Reference Díaz-Obregón, Lloja-Lozano and Carbajal-Zúñiga58). The administration of pumpkin seed proteins after CCl4 intoxication resulted in significantly reduced activity levels of lactate dehydrogenase, alanine transaminase, aspartate transaminase and alkaline phosphatase and hence this protein administration was effective in alleviating the detrimental effects associated with protein malnutrition(Reference Nkosi, Opoku and Terblanche59). Analgesia and anti-inflammation activities were observed with the head of the pumpkin stem(Reference Wang60). Protein isolate from pumpkin seeds could inhibit trypsin and activated Hageman factor, a serine protease involved in blood coagulation(Reference Krishnamoorthi, Gong and Richardson61, Reference Dannenhoffer, Suhr and Thompson62). A dietetic formula made of pumpkin, rice, chicken and vegetable oils was found to be beneficial for children with diarrhoea(Reference Hernández-Ramírez and Guerra-Modernell63). Pumpkin has been used for various cosmetic applications such as skin scrubber, body masque, body butter, massage oil, massage lotion and dry facial masque.
Conclusion and future perspectives
Pumpkin is an edible food which can be included in our daily diet that can give various health benefits to improve our overall health. Pumpkin has various effects beneficial to health such as anti-diabetic, anti-carcinogenic, antioxidant and anti-microbial potential. There are other various health-beneficial effects of pumpkin also reported such as inhibition of kidney stone formation, and hypotensive, anti-inflammatory and blood-coagulatory effects. In various studies pumpkin products show synergistic and no-change effects to treat diseased conditions. Since most of the studies have been done either in vitro or in animal models, controlled clinical trials are strongly needed to confirm these health-beneficial effects in human subjects. There are various food products such as snacks, pies, etc available containing pumpkin alone and in combination with other edible supplements such as ginger and various fruits for human consumption. It would be a good idea to follow up the normal consumption effects in human populations of these products in relation to various chronic diseases such as diabetes, cancer and heart diseases. It is very important to analyse various bioactive components from plant and food components; however, very few components have been isolated and characterised from pumpkin. Therefore it might be a good area to explore in this field to isolate, characterise and evaluate various components of pumpkin from different parts, for medicinal functionality.
Acknowledgements
The present review received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
All authors contributed equally to the preparation of this paper.
There is no conflict of interest for the present study.






