Introduction
Madagascar is a global hotspot for biodiversity conservation and 92% of its 340 reptile species are endemic to the island (Goodman & Benstead, Reference Goodman and Benstead2005). The island has three endemic chameleon genera, and most Brookesia and Calumma species are considered to be forest specialists and are therefore threatened by habitat degradation and fragmentation (Raselimanana & Rakotomalala, Reference Raselimanana, Rakotomalala, Goodman and Benstead2003). The deciduous forests in western Madagascar are subject to chronic degradation both inside and outside protected areas, threatening a number of endemic species, including chameleons (Smith et al., Reference Smith, Horning and Moore1997; Harper et al., Reference Harper, Steininger, Tucker, Juhn and Hawkins2007). It is therefore important to understand how chameleons respond to forest degradation so that conservation resources can be directed to the most vulnerable species.
The chameleon fauna of western Madagascar is generally less well known than that of the eastern forests but the Parc National Tsingy de Bemaraha is an exception (Schimmenti & Jesu, Reference Schimmenti and Jesu1996; Jesu et al., Reference Jesu, Mattioli and Schimmenti1999). Activities that threaten chameleons in the Park include overgrazing by cattle, bushfires, removal of trees, and agricultural expansion. In addition to this, commercial reptile collection is believed to occur although its extent is difficult to ascertain.
Brookesia perarmata is endemic to the Park and is categorized as Vulnerable on the IUCN Red List (IUCN, 2007), is on CITES Appendix I, and is strictly protected from collection under Malagasy law. There are two other restricted range chameleons found in the area, Brookesia exarmata and Furcifer nicosiai, which have yet to be evaluated for the IUCN Red List but are nevertheless considered priority taxa by the Park's conservation team. Information is needed to identify the most important forest areas for these chameleons to assist the Park's conservation and monitoring plan. We compared chameleon densities and habitat use in five different forests in the Park to inform the location of priority conservation sites and the potential impact of forest degradation.
Study area
The study was conducted in the north of Parc National Tsingy de Bemaraha, Fivondronana Antsalova, Melaky Region, Mahajanga Province (Fig. 1). The area is characterized by limestone karst geology. The climate is tropical, with well defined dry (June–October) and wet (November–May) seasons. Maximum monthly rainfall occurs from January to March. A number of vertebrate species are endemic to the Park and it is of growing importance as a tourist destination (Rasoloarison & Paquier, Reference Rasoloarison, Paquier, Goodman and Benstead2003).
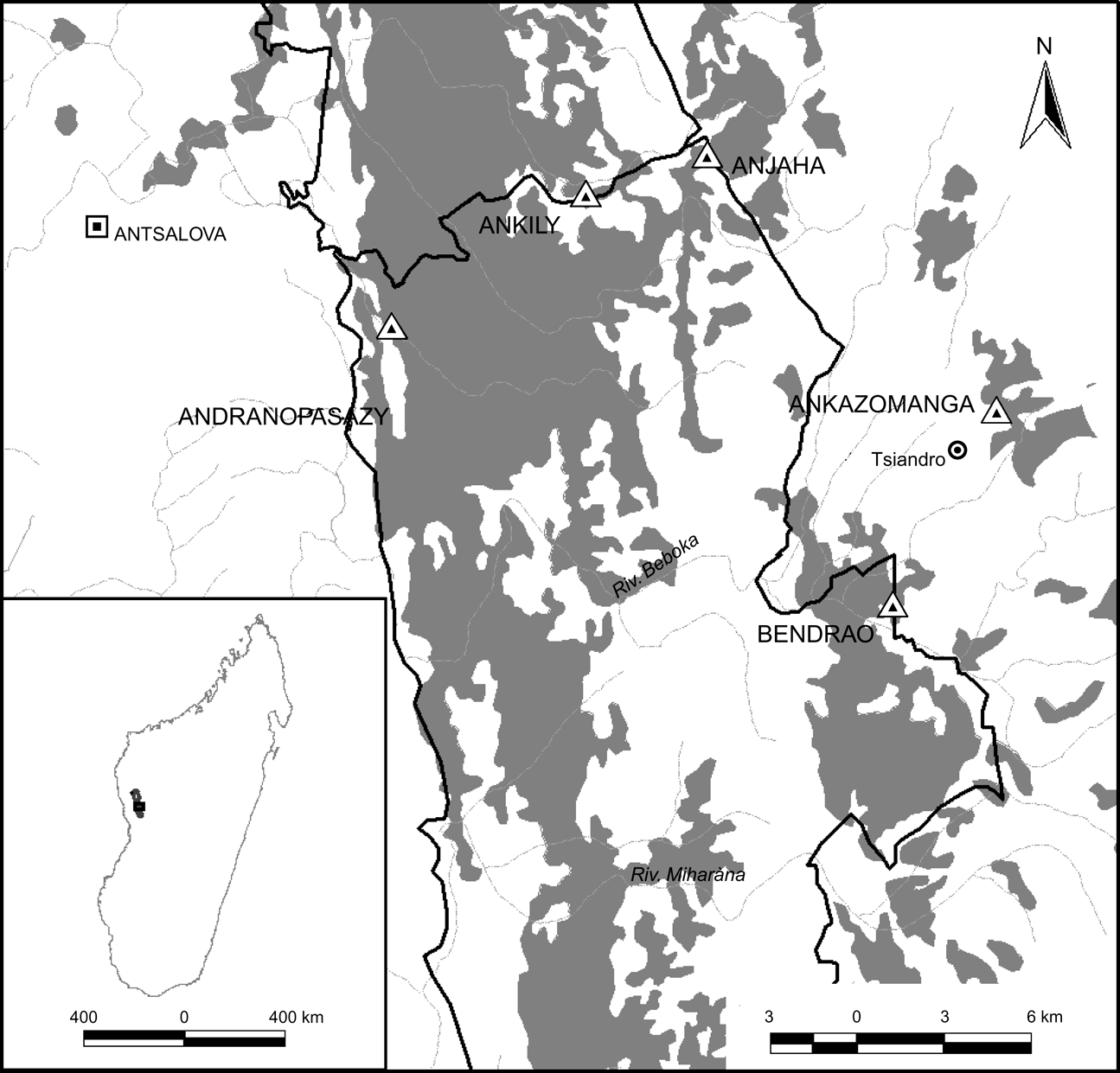
Fig. 1 Location of the study sites (triangles) in the five surveyed forest areas (Table 1) within and around Parc National Tsingy de Bemaraha Réserve/Naturelle Intégrale des Tsingy de Bemaraha (thick solid line), Madagascar; the shading represents areas of deciduous forest. The shaded area on the inset indicates the location of the main map in Madagascar.
Methods
We selected five forest sites in the Park (Fig. 1) across a range of disturbance levels from relatively intact to highly disturbed forest (Table 1). We generally surveyed with two teams per night, each consisting of two people, and with team composition rotating nightly (Table 1). Transects were located randomly in the forest using existing trails as access points and we completed three 50 m length transects each night. The three lines were demarcated the day before the survey and were c. 20 m apart. We adopted the methods previously described for surveying chameleons in Madagascar and used the software Distance v. 4.1 to calculate density (Buckland et al., Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001; Thomas et al., Reference Thomas, Laake, Strindberg, Marques, Buckland and Borchers2004); for more details on survey methodology see Brady & Griffiths (Reference Brady and Griffiths1999).
Table 1 The geographical coordinates, altitude and status of the five surveyed forest sites (Fig. 1), and the survey dates and effort in man hours (based on a standard team of two people spending 2 hours per night) at each site.

Chameleons are active during the day, when they are highly cryptic and difficult to observe, but at night they roost on plant parts above the ground and their pale colouration affords easy detection using torch light. Two surveyors with head torches searched a flank each side of the transect line. We measured the perpendicular distance from the transect line to each chameleon, as well as the height of the roost perch. We marked a sample of chameleon perches each night with a coloured tag and returned the following morning and placed a quadrat (5 × 5 m) on the ground with the perch at the centre. In the quadrat we counted the number of standing, fallen and cut trees, and visually estimated the percentage of open litter and understorey cover. Also, using a 1 m stick marked with cm gradations, we recorded whether there was contact with ground vegetation in four height categories (0–0.24, 0.25–0.49, 0.50–0.74 and 0.75–1.0 m) every 10 cm along two 5 m lines with the perch at the centre. An emergent tsingy (local name for a distinctive, sharp limestone protrusion) index was calculated by multiplying the length × width × height of all bare rock and dividing by the number of distinct rock patches in each quadrat. We also assessed the habitat of two random quadrats per transect line in areas where no chameleons were located during the night, giving us data from areas with and without roosting Brookesia.
We used non-parametric statistics on chameleon abundance to test for differences between forest sites. We were unable to calculate abundance indices because of small sample sizes for some sites (Jenkins et al., Reference Jenkins, Brady, Bisoa, Rabearivony and Griffiths2003). ANOVA was used to test for differences between habitat features at each site. ANOVA comparisons were made between quadrats with and without chameleons, and post-hoc tests (Fisher's PLSD) indicated which factor was significantly different.
Results
We found a total of 758 chameleons (734 Brookesia and 24 Furcifer). The most frequently encountered species was Brookesia brygooi (n = 444), followed by B. perarmata (192), B. exarmata (98), F. nicosiai (22) and F. cf. petteri (2). The density of B. brygooi was the highest across sites (53.2 ha-1, coefficient of variation, CV, 8.1%), followed by B. perarmata (29.15 ha-1, CV 17.1%) and B. exarmata (18.74 ha-1, CV 21.6%). F. nicosiai was only found at low densities (1.50 ha-1, CV 25.11%). There were clear differences in density of Brookesia between the five sites (Table 2). Ankazomanga forest contained no B. perarmata, a low density of B. exarmata and the highest density of B. brygooi. Of the four sites with three sympatric Brookesia species the highest densities were recorded at Bendrao. F. nicosiai was encountered too infrequently for detailed comparisons but was recorded at all sites except Bendrao.
Table 2 Number, abundance and density (with % coefficient of variation, CV, and 95% confidence interval, CI) of the three Brookesia chameleon species recorded in the five surveyed forest sites (Fig. 1, Table 1) within and close to Parc National Tsingy de Bemaraha, and df and model type used for determination of density using Distance.

There were significant differences in the abundance of the Brookesia species between the five sites (Kruskal Wallis df = 4; B. perarmata H = 61.4, P < 0.001; B. exarmata, H = 73.3, P < 0.01; B. brygooi, H = 48.7, P < 0.001). Patterns in abundance were similar to density, with B. brygooi most abundant at Ankazomanga where the other two species were absent or uncommon. B. exarmata and B. perarmata were most abundant in Bendrao (Table 2).
There were significant habitat differences between quadrats around chameleon roosts and randomly placed quadrats (Table 3). Roosting B. perarmata were typically found in areas with well-developed leaf litter and understorey and few emergent tsingy rocks. B. exarmata roosts were associated with emergent tsingy rocks but also an open leaf litter layer and low understorey cover. Roosting areas used by B. brygooi were found in disturbed areas with cut trees and a tall vegetation layer.
Table 3 ANOVA (F) comparisons and mean (± SE) habitat characteristics recorded in quadrats either with or without roosting Brookesia in and close to Parc National Tsingy de Bemaraha (Fig. 1, Table 1). Differences in six pair-wise comparisons in post-hoc tests are indicated by superscript lower case letters, where the same letter indicates that the habitat characteristic was significantly different.
*P < 0.05; **P < 0.001
Discussion
The Parc National Tsingy de Bemaraha is an important site for reptile conservation in Madagascar and is the only known location for B. perarmata. There are no published records of B. perarmata outside the Park, although a report cited in Schimmenti & Jesu (Reference Schimmenti and Jesu1997) of it occurring in another forest in the region needs to be investigated further. We found B. perarmata in the four sites within the Park but not in Ankazomanga, a degraded forest 10 km from the Park's boundary. B. exarmata is also thought to be endemic to the karst forests of the Park (Schimmenti & Jesu, Reference Schimmenti and Jesu1996) but we cannot rule out the possibility that it also occurs in other forests in the region where suitable habitat occurs. B. brygooi has a relatively wide distribution in western Madagascar (Raxworthy & Nussbaum, Reference Raxworthy and Nussbaum1995), over 17–23°S, and it was ubiquitous in the forests of northern Parc National Tsingy de Bemaraha; the species is restricted to deciduous forest and is thought to tolerate a wide range of environmental conditions (Carpenter & Robson, Reference Carpenter and Robson2005).
Brady & Griffiths (Reference Brady and Griffiths1999) highlighted the problem of small sample sizes and the associated high errors in the resulting density estimates calculated with Distance, and suggested that a coefficient of variation of < 30% was appropriate for calculating reliable density estimates of forest Calumma chameleons in Madagascar. Seven of our density estimates yielded a coefficient of variation of ≤ 30% and this was achieved with 34–104 observations per species. Density estimates based on < 10 individuals need to be interpreted with due caution.
Differences in the density of chameleons between sites may be related to vegetation structure/habitat quality, illicit collection (past or present), seasonality or altitude. Although we sampled sites sequentially, visits were short (mean 9.4 days) with only a mean of 2.8 days between sites, and even though we cannot rule out a relationship between chameleon abundance and the progression of the rainy season, long-term climatic data from Antsalova (ANGAP(Association Nationale pour la Gestion des Aires Protégées), unpubl. data) demonstrates higher monthly precipitation in January, February and March than December or April, thus justifying our study period.
Altitude is potentially an important factor because other Brookesia species are often restricted to narrow altitudinal ranges (Raxworthy & Nussbaum, Reference Raxworthy and Nussbaum1995; Glaw et al., Reference Glaw, Vences, Ziegler, Bohme and Kohler1999), although usually in montane sites with a wider altitudinal span than in our study. B. perarmata density and abundance increased with altitude but the species was not recorded at the highest elevation in Ankazomanga (497–563 m). Future surveys should focus on intact forest at 500 m to determine whether the absence of B. perarmata from Ankazomanga is related to elevation as well as habitat structure.
Brookesia habitat preferences are poorly known, with knowledge generally based on the collection of a few individual animals (Raxworthy, Reference Raxworthy1991; Raxworthy & Nussbaum, Reference Raxworthy and Nussbaum1995; Glaw et al., Reference Glaw, Vences, Ziegler, Bohme and Kohler1999). There is evidence that certain Brookesia are intolerant of severe habitat modification (Jenkins et al., Reference Jenkins, Brady, Bisoa, Rabearivony and Griffiths2003) and, although a few taxa have been found in degraded sites (Glaw et al., Reference Glaw, Vences, Ziegler, Bohme and Kohler1999), they are generally considered to be dependent on relatively intact forest (Carpenter & Robson, Reference Carpenter and Robson2005). Although all three Brookesia species coexisted at four forests in our study there were inter-specific differences in the habitat associated with nocturnal perches. B. exarmata roosts were associated with areas of forest that had relatively little understorey but a high leaf litter cover and notable surface area covered by emergent tsingy rock. Schimmenti & Jesu (Reference Schimmenti and Jesu1996) reported B. exarmata from dense sub-humid forest but this was based on only a small number of observations. B. perarmata was also associated with open areas but with a more developed understorey layer. Overgrazing by cattle and fire are therefore potential threats to these two Brookesia species because of the damage to leaf litter, loss of native understorey and colonization of pioneer species. Although little is known about the diurnal foraging of Brookesia there are significant differences in nocturnal perch height between sympatric species in Parc National Tsingy de Bemaraha (Randrianantoandro et al., Reference Randrianantoandro, Randrianavelona, Andriantsimanarilafy, Fideline, Rakotondravony and Jenkins2007) and changes to vegetation structure are therefore likely to affect these chameleons.
The Park has a permanent ANGAP (Association Nationale pour le Gestion des Aires Protégées) team with field officers who regularly patrol the forest and conduct nocturnal monitoring of Brookesia between January and March. It would also be helpful to install permanent vegetation plots in the areas monitored for Brookesia to follow any long-term changes in vegetation structure. Illegal collection of reptiles from the Park is more difficult to monitor.
B. perarmata was most common in Bendrao where it was sympatric with B. exarmata and B. brygooi. As the most intact forest and with the highest densities of priority chameleon species we recommend that particular effort is given to conserving this site. However, this was the only site in which we did not locate F. nicosiai; this may also be related to forest structure because Furcifer chameleons are generally associated with open habitats or forest edges (e.g. Andreone et al., Reference Andreone, Guarino and Randrianirina2005; Rabearivony et al., in press). At Anjaha the forest inside the Park boundary was disturbed by people but some areas of the unprotected buffer zone were relatively undisturbed and require additional conservation measures to prevent the removal of timber.
Carpenter & Robson (Reference Carpenter and Robson2005) reported that 2,215 B. perarmata were exported to the USA between 1996 and 2001 but that numbers dropped to three by 2003 following its inclusion on Appendix I of CITES. Under Malagasy law the collection of B. perarmata from Parc National Tsingy de Bemaraha is illegal, yet anecdotes of reptile collection from the Park continue. Personnel from ANGAP and the Malagasy government began checking passenger baggage on Air Madagascar flights departing from Antsalova, west of the Park, in 1997. Consignments of B. perarmata were intercepted in 1998 and 2000, and one of these contained 250 alive and 10 dead chameleons hidden inside a radio cassette player. Better coordination is needed between the authorities in major towns east of the Park to develop a more comprehensive strategy for monitoring illegal trade.
Acknowledgements
This work was funded by the Darwin Initiative, UK, and Calumma Ecological Services, UK. We are grateful to Professor Olga Ramilijaona for assisting our research, which was authorized by the Ministry of Environment, Water and Forests. We received invaluable support from ANGAP staff (R. Rindra, Gaston, Julien, Vinola, Raymond, Momo, Honoré, Solofo, Donné, Georges, and Antoine) and benefited greatly from the support of the Programme Bemaraha team in the Antsalova office. We are also grateful to Dr Frank Glaw for verifying our identifications. Dr Nik Cole and an anonymous referee helped to improve the manuscript.
Biographical sketches
J. Christian Randrianantoandro has studied chameleons since 1998 and is currently developing monitoring protocols for chameleons in protected areas in western Madagascar. Roma Randrianavelona has also studied Zonosaurus plated-lizards, and is particularly interested in working with communities to conserve Mantella frogs. Raphali R. Andriantsimanarilafy is a student at the University of Toliara and is focusing on the biogeography of reptiles in karst forest. Elisoa Hantalalaina is a student at the Université d'Antananarivo and is studying the ecology of forest chameleons. Daniel Rakotondravony is the head of the Département de Biologie Animale, Université d'Antananarivo and specializes in the conservation of small mammals. Hery Lala Ravelomanantsoa is the director of the Tsingy de Bemaraha National Park. Mamy Randrianasolo coordinates the conservation and monitoring activities in the Park. Richard K.B. Jenkins coordinates projects to conserve Malagasy endemic vertebrates and their habitats, with current efforts focusing on bats, chameleons, amphibians and bushmeat.






