Introduction
Sharks, rays, and skates belong to the cartilaginous fish (Chondrichthyes), forming a monophyletic group, the Neoselachii sensu Compagno (Reference Compagno1977) or Elasmobranchii sensu Maisey (Reference Maisey2012). So far, more than 500 species of sharks have been described, which amounts to more than 40% of all known extant chondrichthyans (Ebert et al. Reference Ebert, Fowler and Compagno2013; Weigmann Reference Weigmann2016). Although they are low in number compared with bony fishes, sharks represent a diverse group, occupying many different ecological niches and trophic levels—from zooplanktivorous species at low trophic levels (e.g., whale shark Rhincodon typus), to secondary consumers (e.g., nurse shark Ginglymostoma cirratum), and third-order consumers (e.g., white shark Carcharodon carcharias and tiger shark Galeocerdo cuvier), which is similar to marine mammals (Cortès Reference Cortès1999). They occur in both marine and freshwater environments, ranging from the surface to more than 4000 m depth and are distributed worldwide, from arctic waters (down to 0.6°C water temperature) to tropical waters (Ebert et al. Reference Ebert, Fowler and Compagno2013). Therefore, this group, which survived several mass extinction events since its first appearance in the fossil record, can be considered very successful despite the rather small taxonomic diversity.
Despite their obvious evolutionary and ecological success, an increasing number of shark species are threatened with extinction as a result of overfishing, habitat degradation, and climate change. Currently we are facing an anthropogenically driven mass extinction event, very similar to past mass extinctions, with global extinction rates being elevated up to a thousand times higher than former extinction events as shown by the fossil record (Pimm et al. Reference Pimm, Russell, Gittleman and Brooks1995; Ceballos et al. Reference Ceballos, Ehrlich and Dirzo2017). Compared with most other vertebrates, the extinction risk for chondrichthyans is substantially higher, large-bodied and shallow-water species are especially at greatest risk, and overall, only one-third of all chondrichthyan species can be regarded as safe (Dulvy et al. Reference Dulvy, Fowler, Musick, Cavanagh, Kyne, Harrison, Carlson, Davidson, Fordham and Francis2014; Stein et al. Reference Stein, Mull, Kuhn, Aschliman, Davidson, Joy, Smith, Dulvy and Mooers2018).
While several iconic sharks such as the white shark attracted a large proportion of scientific interest in recent years, the tiger shark and its evolutionary history remained largely ignored. Due to their tremendous size of up to 5.5 m (Holmes et al. Reference Holmes, Sumpton, Mayer, Tibbetts, Neil and Bennett2012), their broad and heavily mineralized jaws (Moss Reference Moss1972), and their specialized teeth, tiger sharks are capable of preying on a wide variety of different food items, including sea turtles, birds, marine mammals, and even garbage (Gudger Reference Gudger1949; Compagno Reference Compagno1984; Randall Reference Randall1992; Kent Reference Kent2018).
A well-founded knowledge of the fossil record of Galeocerdo and its nominal species is important for diversity and disparity analyses through geologic time to better understand how vulnerable the modern tiger shark is to current threats. It is, however, represented entirely by isolated teeth, because the skeleton consists predominantly of cartilage, which fossilizes only under very specific conditions, rendering the identification of fossil taxa difficult. The oldest occurrences of tiger sharks were reported from the Ypresian, early Eocene (ca. 56–47.8 Ma; Cappetta Reference Cappetta1981, Reference Cappetta and Schultze1987). In the last 200 years, more than 60 fossil Galeocerdo species were described based on isolated teeth and even scales, many of which meanwhile were reassigned to other genera and species. This resulted in 23 valid species, some of which are considered doubtful, depending on authors (see Supplementary Material). Based on these records, an increase in diversity from the Paleogene (Eocene–Oligocene with 8 species) to the Neogene–Quaternary (Miocene–Holocene with 15 species) seemingly occurred, before all but 1 species went extinct in the late Miocene to Pliocene (Fig. 1).
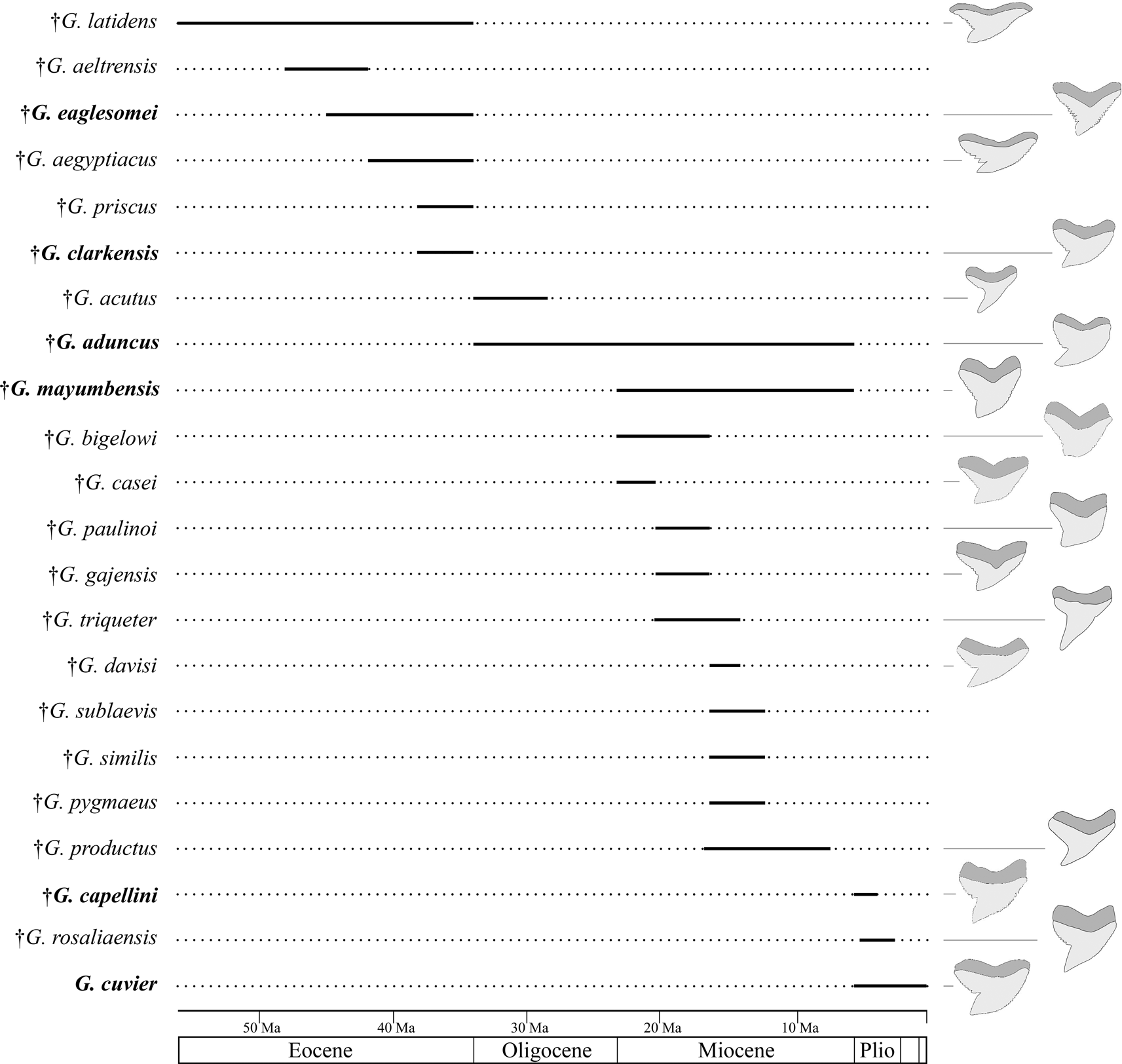
Figure 1. First and last occurrence of nominal species of the genus Galeocerdo (see Supplementary Material). †Galeocerdo subcrenatus is not listed, as no formation is indicated in the original description (see Emmons Reference Emmons1858). The species considered valid in this study are written in bold.
The high morphological similarity of extinct tiger shark teeth led to a poorly understood evolutionary history of this unique genus, which is of great ecological importance. The goal of the present study is to revise the fossil record of the extant G. cuvier and the extinct members of the tiger shark lineage by applying geometric morphometric techniques. Furthermore, teeth of Galeocerdo are compared with genera exhibiting superficially similar-looking teeth, that is, Hemipristis and †Physogaleus, to validate the applicability of this method to separate similar-appearing teeth of different genera.
Significant morphological inter/intraspecific variations and similarities between teeth of extinct and extant tiger shark species were examined and were used in combination with qualitative (morphological) characters to evaluate and establish the validity of extinct tiger shark species and to revise the fossil record of the extant species. Additionally, short morphological descriptions of the 23 previously recognized extinct and extant tiger shark species are provided (see Supplementary Material).
Material and Methods
Material
Isolated teeth of extinct and extant shark species as well as dried jaws of the extant tiger shark Galeocerdo cuvier were used in this study. A dagger preceding the name identifies extinct species in the text as well as in all tables and figures.
The sample consists in total of 569 shark teeth, photographed in labial view. Eighteen published illustrations of tiger shark teeth were used if insufficient or no other material of the corresponding species was available. The majority of the teeth (n = 450) belongs to tiger sharks and is represented by the 16 nominal species: †G. acutus, †G. aduncus, †G. aegyptiacus, †G. bigelowi, †G. capellini, †G. casei, †G. clarkensis, G. cuvier, †G. davisi, †G. eaglesomei, †G. gajensis, †G. latidens, †G. mayumbensis, †G. paulinoi, †G. rosaliaensis, and †G. triqueter. However, teeth of morphologically similar species of Hemipristis (†H. curvatus and †H. serra) and †Physogaleus (†P. alabamensis and †P. contortus) were also included. Five species were represented with their holotype (†G. casei, †G. davisi, †G. gajensis, †G. paulinoi, †P. alabamensis) and three with the whole type series (†G. clarkensis, †G. eaglesomei, †G. rosaliaensis). Detailed information about the material (e.g., provenance, geologic age) is depicted in Supplementary Table 1.
Geometric Morphometrics
The tooth shape of fossil and extant Galeocerdo species as well as Hemipristis and †Physogaleus was studied with 2D landmark-based geometric morphometrics. Three homologous landmarks were digitized using the software tpsDIG2 (v. 2.31; Rohlf Reference Rohlf2017). Additionally, 64 semilandmarks were digitized between the homologous landmarks to capture the overall tooth shape (Fig. 2).
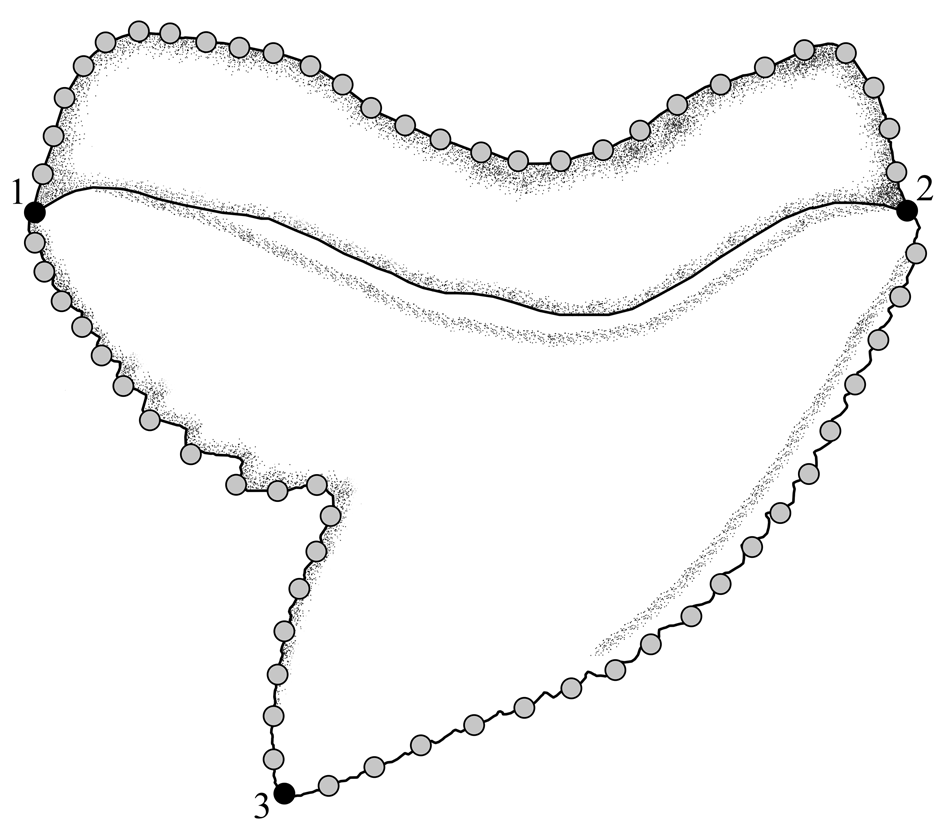
Figure 2. Location of the landmarks and semilandmarks for the geometric morphometric analyses. The landmarks are located on the (1) base of distal cutting edge, (2) base of mesial cutting edge, and (3) tip of cusp. Twenty-eight semilandmarks are located along the outline of the root between the base of the distal and mesial cutting edge, 18 are located between the base of the mesial cutting edge, and the tip of the cusp and 18 semilandmarks are situated between the tip of the cusp and the base of the distal cutting edge.
To minimize the variance caused by size, orientation, location, and rotation, a generalized Procrustes analysis (GPA) was performed on the landmark coordinates. The sliding semilandmarks were allowed to slide to minimize the bending energy (Gunz and Mitteroecker Reference Gunz and Mitteroecker2013). The aligned coordinates were then subjected to a principal component analysis (PCA) to assess shape variation of teeth. Tooth shape differences between genera and within Galeocerdo species were estimated with a permutational analysis of variance (ANOVA), followed by pairwise comparisons between the groups, with the functions procD.lm and pairwise considering the distances between means in the R packages geomorph (v. 3.1; Adams et al. Reference Adams, Collyer, Kaliontzopoulou and Sherratt2016) and RRPP (Collyer and Adams Reference Collyer and Adams2018).
Disparity through Time
To evaluate how the dental morphological disparity through geologic time changed among Galeocerdo species, we assigned taxa to the time bins Eocene, Oligocene, Miocene, Pliocene, and Holocene. We used the Procrustes variance (Zelditch et al. Reference Zelditch, Swiderski and Sheets2012), applied the function morphol.disparity in the R package geomorph (v. 3.1; Adams et al. Reference Adams, Collyer, Kaliontzopoulou and Sherratt2016), and performed post hoc pairwise comparisons considering the variance between the epochs to estimate differences between them.
Qualitative Reevaluation of Morphological Characters
Although geometric morphometrics is a useful method to quantify shape and morphology, some qualitative characters are not taken into account with this technique. In shark teeth, such features include the presence of serrations on the cutting edges as well as their quality (e.g., coarse, compound, minor), characters that are often essential for identifications on the species level. To enable a best possible determination of the teeth included in the present study, all species were examined and described, incorporating diagnostic characters from first descriptions and additional literature (see Supplementary Material). The tooth terminology used broadly follows Cappetta (Reference Cappetta and Schultze2012).
Results
PCA on the Whole Sample
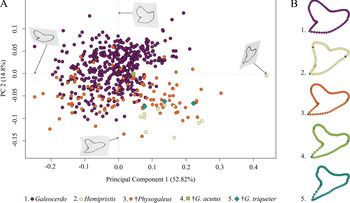
This analysis contained a total of 569 specimens, representing the three genera Galeocerdo, Hemipristis, and †Physogaleus (see Supplementary Table 1) and resulted in 134 PC axes with the first four explaining 83.9% of the total morphological variation. All remaining axes each account for less than 5% of the variance. PC 1 (52.82%) describes a morphological shift from broad and labiolingually compressed teeth with a flattened root and a strongly distally inclined cusp on the negative side to high teeth having a V-shaped root and nearly vertical distal and mesial cutting edges and lacking a distinct distal notch on the positive side. Positive scores of PC 2 (14.8%) are related to very broad and bulky teeth with an asymmetrical root, a strongly curved mesial cutting edge, a deep distal notch, and a convex distal heel. Negative scores indicate slenderer teeth with a symmetrical root, a less-curved mesial cutting edge, a more obtuse-angled notch, and a straight distal heel (Fig. 3).
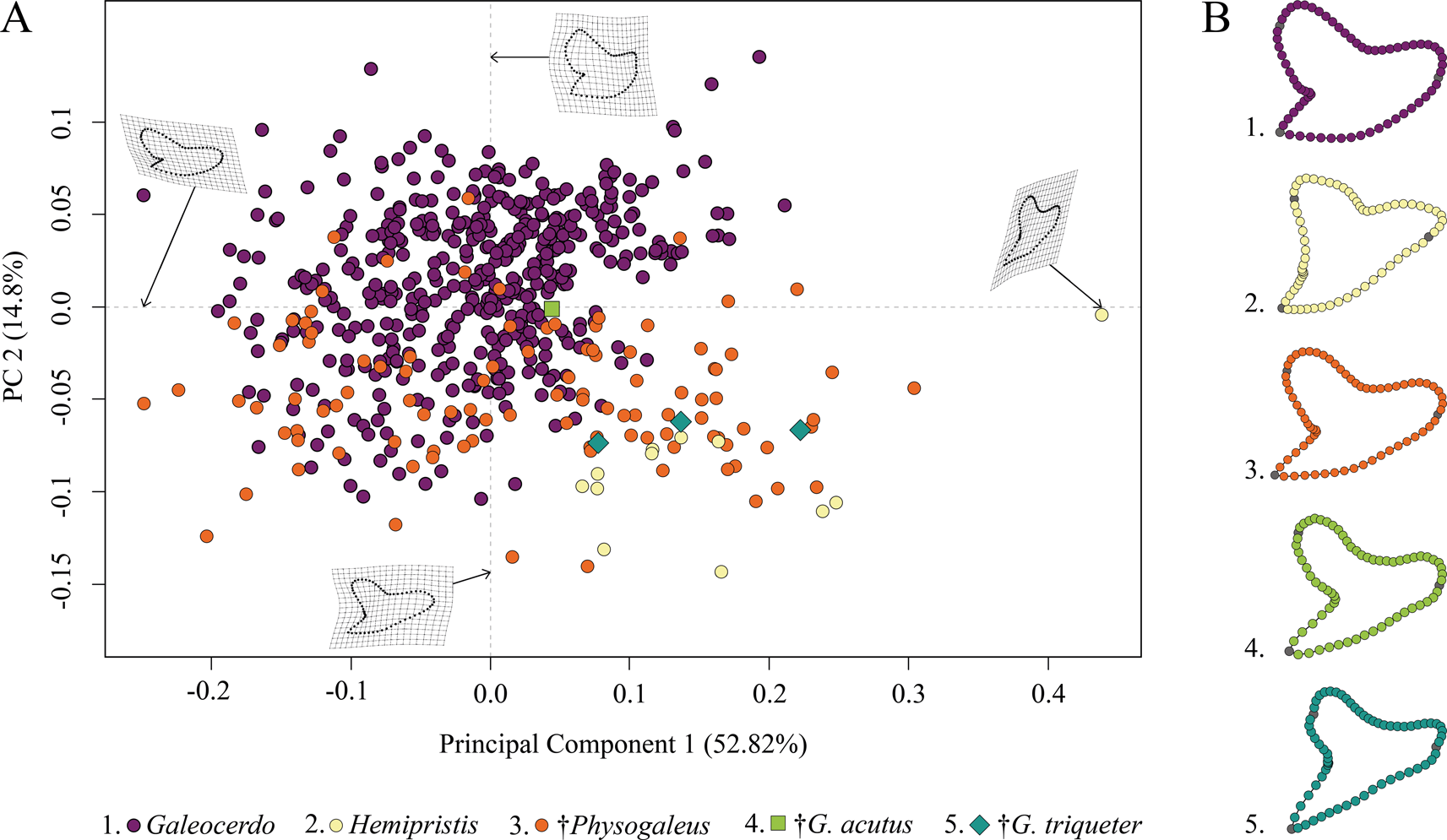
Figure 3. Morphospace of all examined shark teeth, divided into the three genera Galeocerdo, Hemipristis, and †Physogaleus, with the two species †G. acutus and †G. triqueter highlighted. A, Scatter plot of the first two principal component (PC) axes. B, Mean tooth shapes of all examined groups.
The morphospace occupied by Hemipristis is completely separated from Galeocerdo, whereas the morphospace of †Physogaleus overlaps with both Galeocerdo and Hemipristis.
Two doubtful species, the Oligocene †G. acutus and the Miocene †G. triqueter (represented by one and three teeth, respectively), were included to determine their genus affiliations. According to our results, †G. acutus plots within the overlapping area of †Physogaleus and Galeocerdo, while †G. triqueter is located exclusively within the morphospace of †Physogaleus (Fig. 3). Morphological similarities of both species with †Physogaleus contortus include slender and smooth/minor serrated tooth crowns and bulky roots, supporting their assignment to the genus †Physogaleus (Fig. 3B, Supplementary Material).
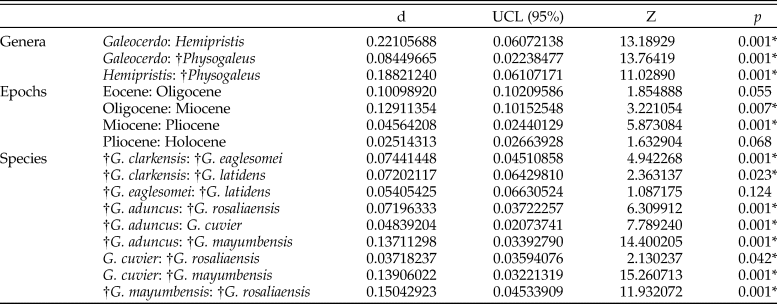
The implemented permutational ANOVA (PERMANOVA) reveals significant differences in shape among Galeocerdo, Hemipristis, and †Physogaleus (Table 1). A pairwise comparison further corroborates the separation of all three genera (Table 2).
Table 1. Results of the permutational analysis of variance to test for differences in tooth shape between the examined groups. An asterisk indicates a p-value < 0.05. SS, sum of squares; MS, mean squares.
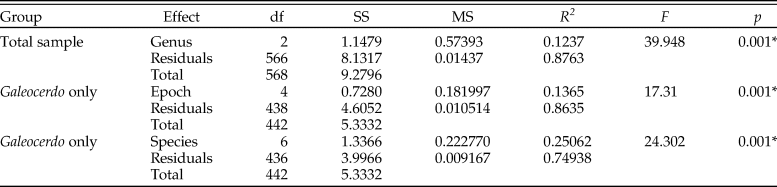
Table 2. Results of the pairwise comparison to test for differences in tooth shape between the examined groups. Signficance is depicted as p-value (an asterisk indicates a p-value < 0.05). d, distance; UCL, upper confidence limit; Z, Z-score.
Tiger Shark Species Shape Variation
This analysis contained a total of 450 Galeocerdo specimens, assigned to the nominal species †G. aduncus, †G. bigelowi, †G. capellini, †G. casei, †G. clarkensis, G. cuvier, †G. davisi, †G. eaglesomei, †G. gajensis, †G. latidens, †G. mayumbensis, †G. paulinoi and †G. rosaliaensis (see Supplementary Table 1).
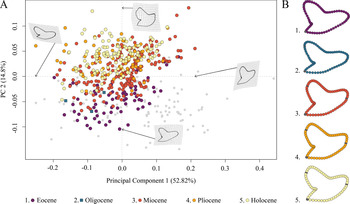
The species distribution of Galeocerdo in the morphospace is related to geologic time and shows a separation of Paleogene from Neogene and younger species along PC 2; Eocene and Oligocene species mainly accumulate in the negative realm, and Miocene species are partly overlapping with both but are better represented by positive values of PC 2. Pliocene and Holocene species, however, are highly overlapping with each other and almost completely separated from Eocene and Oligocene species (Fig. 4).
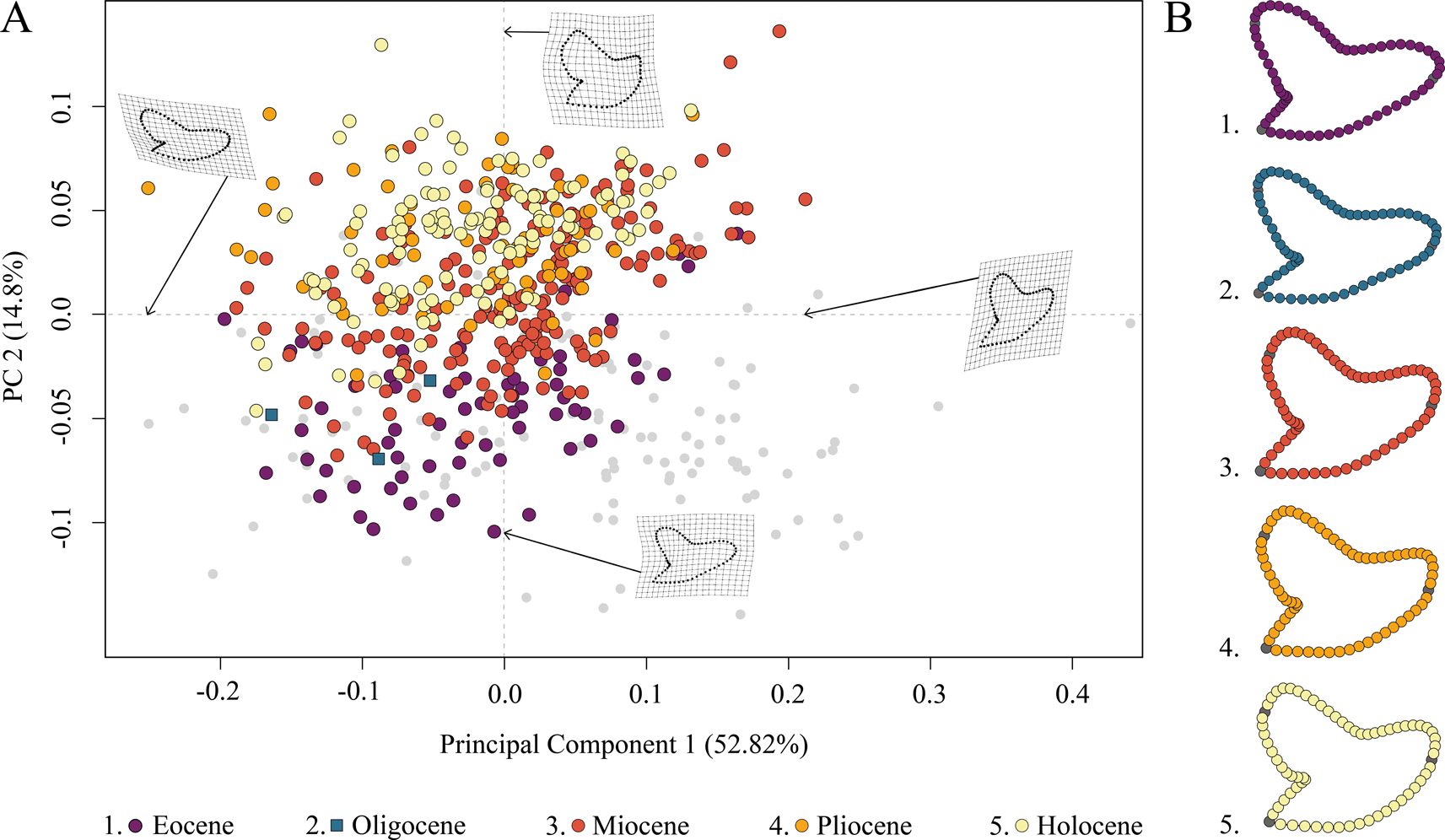
Figure 4. Morphospace occupation of all examined Galeocerdo teeth, divided by epochs. A, Scatter plot of the first two principal component (PC) axes. B, Mean tooth shapes of all examined groups.
PC 1 describes the difference between lateral and anterior teeth, with negative scores indicating broad and compressed teeth with a flattened root and a strongly distally inclined primary cusp. Conversely, positive scores are related to high, upright teeth with a V-shaped and broad root. PC 2 explains the morphological change of teeth from Eocene to younger stratigraphic ages, with negative values indicating teeth having a symmetrical root, a slightly curved mesial cutting edge, an obtuse-angled distal notch, and a straight distal heel. Positive scores are related to broad teeth with strongly curved mesial and distal cutting edges, a bulky crown, and a deep distal notch (Fig. 4).
The implemented PERMANOVA suggests that the examined time-bin compositions are significantly different (Table 1), except for the Eocene/Oligocene association and the Pliocene/Holocene association (Table 2). However, only three specimens from the Oligocene could be included in this study, and therefore no statistically relevant conclusions can be reached here.
Paleogene Tiger Sharks
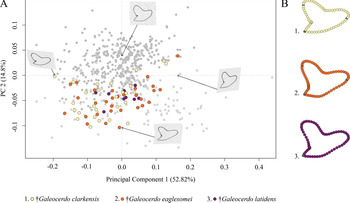
The Paleogene species †G. clarkensis, †G. eaglesomei, and †G. latidens mainly occupy the negative region of PC 1 and PC 2, with †G. clarkensis and †G. eaglesomei being distributed broadly across the shared realm and †G. latidens accumulating in the center (Fig. 5).
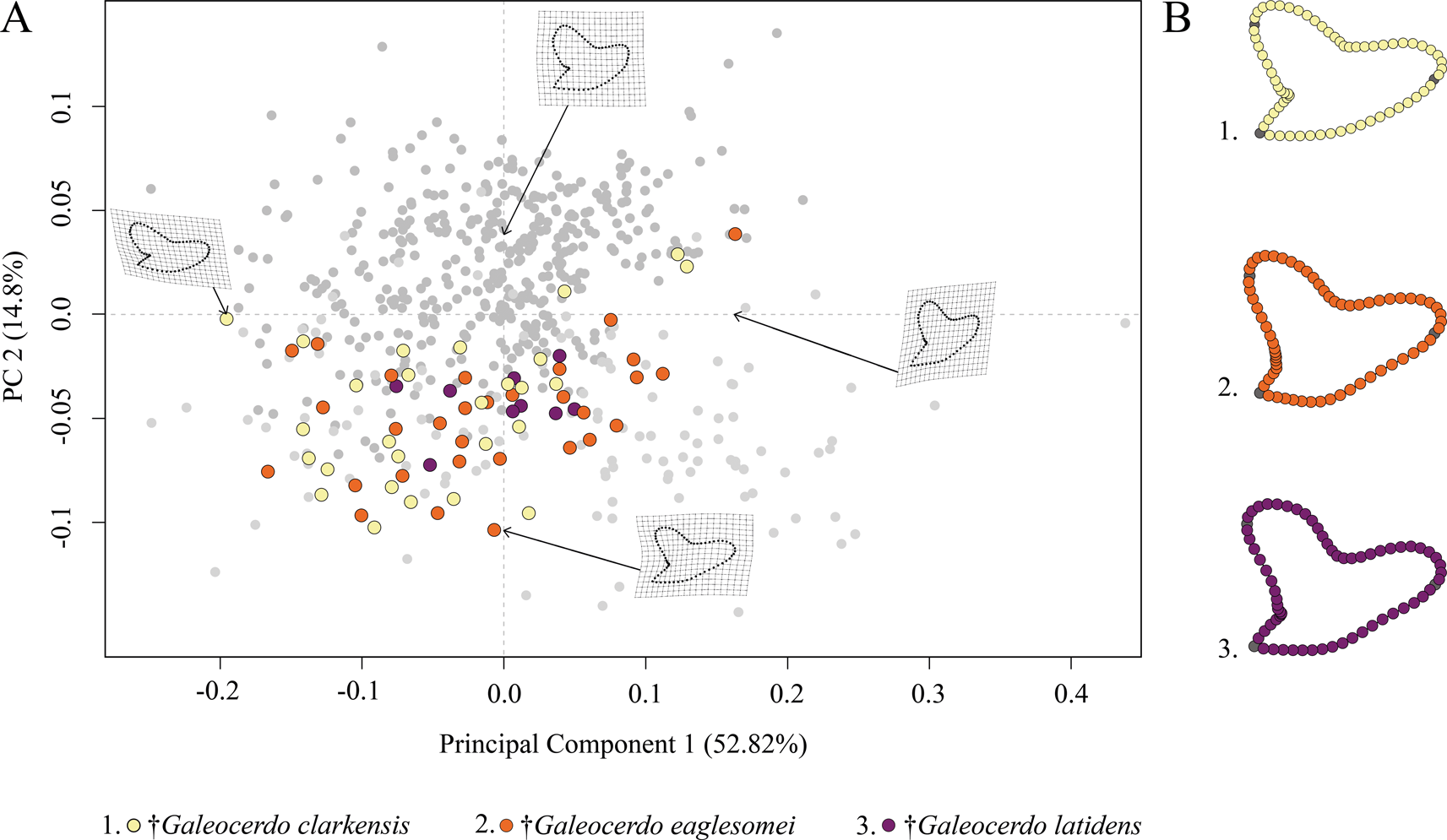
Figure 5. Morphospace of all examined Paleogene Galeocerdo teeth. A, Scatter plot of the first two principal component (PC) axes. B, Mean tooth shapes of all examined groups.
Despite the overlap of the species in the morphospace, a PERMANOVA suggests significant differences between them (Table 1). The pairwise comparison between the species, however, reveals a high degree of overlap between †G. eaglesomei and †G. latidens, indicating no significant differences between these two species (Table 2).
Neogene–Quaternary Tiger Sharks
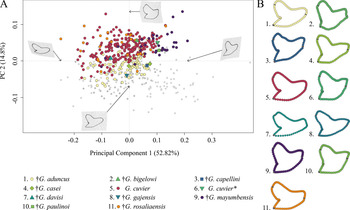
The morphospace occupation of Neogene–Quaternary (Miocene–Holocene) species shows a broad distribution of G. cuvier and †G. rosaliaensis, predominantly in the positive realm of PC 2. †Galeocerdo aduncus is partly overlapping with both species in the morphospace but is distributed mainly along the negative scores of PC 2. †Galeocerdo mayumbensis is most separated from the others and occupies the positive end of PC 1. Six doubtful species (†G. bigelowi, †G. casei, †G. capellini, †G. davisi, †G. gajensis, †G. paulinoi) were additionally included in the morphospace to determine their species affiliations. Accordingly, †G. capellini and †G. gajensis, which are only represented by one tooth each, plot within the morphospace of †G. aduncus. The single tooth of †G. davisi is located within the overlapping morphospaces of †G. aduncus, G. cuvier, and †G. rosaliaensis. The highly similar tooth morphology of †G. bigelowi, †G. casei, and †G. paulinoi to that of †G. mayumbensis is reflected in the morphospace occupation, with †G. bigelowi and †G. paulinoi plotting within and †G. casei plotting extremely close to the morphospace occupied by †G. mayumbensis (Fig. 6).
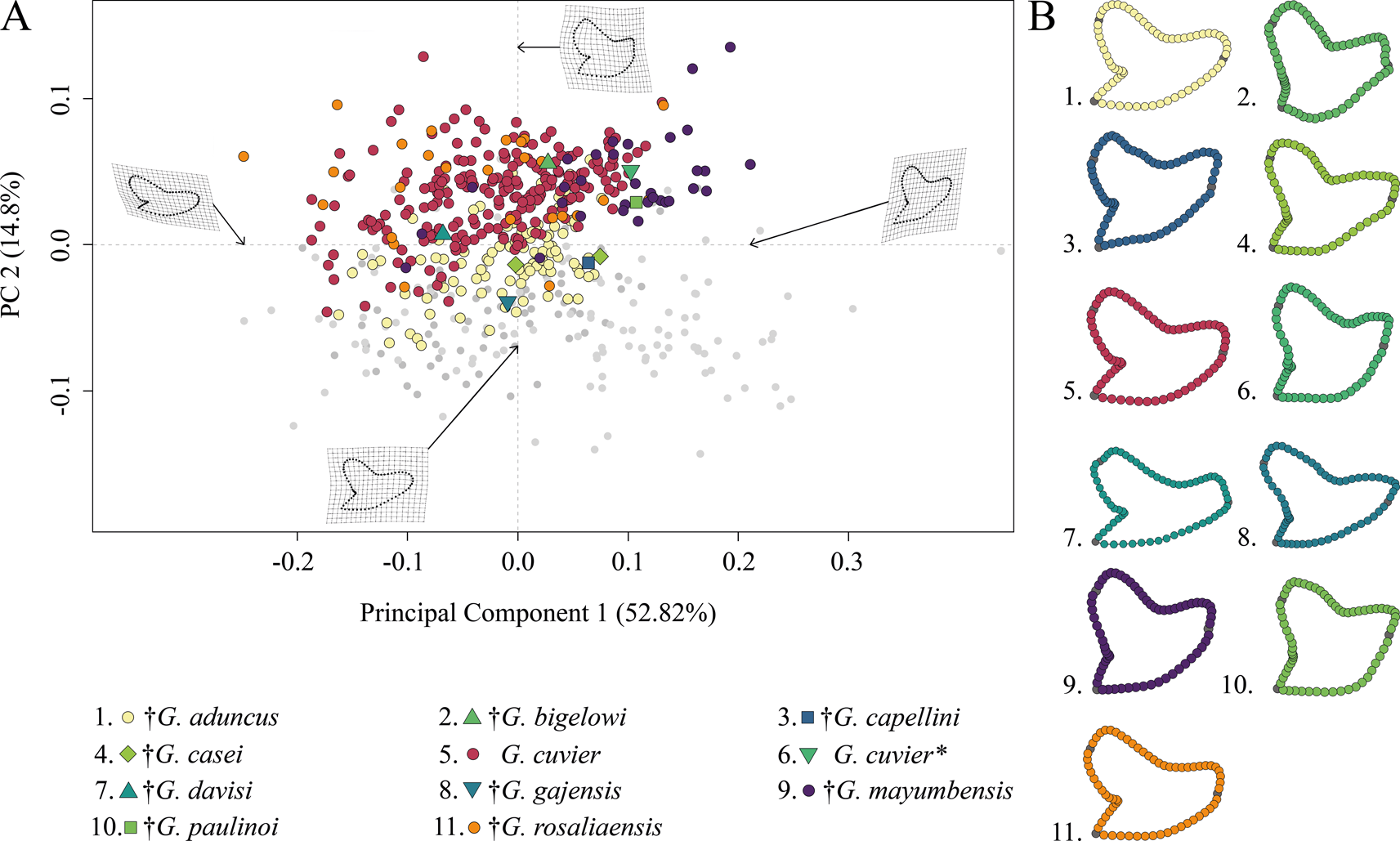
Figure 6. Morphospace of all examined Neogene–Quaternary Galeocerdo teeth. A, Scatter plot of the first two principal component (PC) axes. The single tooth described by dos Reis (Reference dos Reis2005) as G. cuvier is highlighted with an asterisk. B, Mean tooth shapes of all examined groups.
The results of the PERMANOVA indicate a significant difference between the examined species (Table 1). This is further corroborated by a pairwise comparison, in which all species are significantly different from one another (Table 2).
Dental Disparity through Time
The dental morphological disparity of Galeocerdo attains its maximum in the Eocene, followed by a massive drop, resulting in the lowest disparity level in the Oligocene. During the Miocene, disparity increases again, albeit not to the levels attained in the Eocene, and eventually keeps a high level through the remaining Neogene and Quaternary (Fig. 7, Table 3).
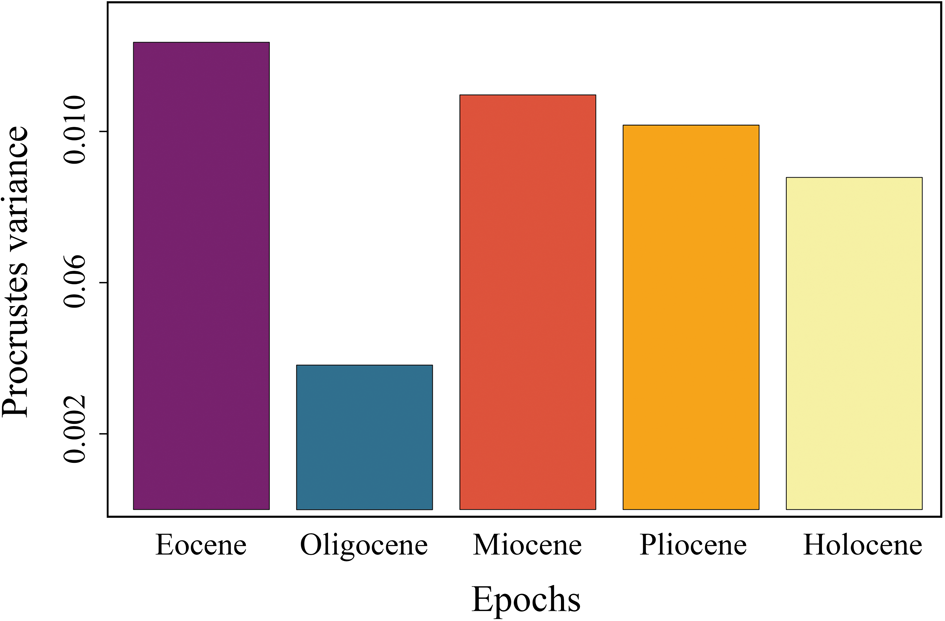
Figure 7. Disparity through time of the dental morphology of Galeocerdo.
Table 3. Results of the morphological disparity through time analysis.
Discussion
Geometric Morphometrics
Up to now, more than 60 extinct tiger shark species based on isolated teeth have been described since the first taxonomic description of the extant species ca. 200 years ago. After various revisions, 23 species currently are considered valid, although many remain dubious. To overcome the problem of qualitative morphological characters that often bear the problem of undetected convergences, we additionally used quantitative geometric morphometric analyses here. Unfortunately, it was not possible to obtain teeth, or at least useful figures, of all 23 species, because six species were described without illustration (i.e., †G. aeltrensis, †G. priscus, †G. pygmaeus, †G. similis, †G. subcrenatus, and †G. sublaevis), and figures of †G. productus were only available in lingual view and we used solely teeth photographed in labial view in the present study to minimize inaccuracies. Nevertheless, we were able to analyze teeth of 16 nominal species (see “Material and Methods” section for detailed account) and compared them with morphologically similar teeth of Hemipristis, †Physogaleus contortus, and †P. alabamensis. Our results clearly show that teeth of Hemipristis, †Physogaleus, and Galeocerdo species are well separated despite dental resemblances, especially between Hemipristis and †G. eaglesomei. It is furthermore evident that two species traditionally included in Galeocerdo, †G. acutus and †G. triqueter, must be included in †P. contortus, although this should be treated with caution because of the low number of specimens, qualitative characters (e.g., slender and smooth/minor serrated tooth crowns) further corroborate this result. Unfortunately, no tooth of †G. productus was available for the quantitative analysis. The characters described by Agassiz (Reference Agassiz1856), such as only a slightly curved cusp and small serrations at the basis, however, are very similar to dental characters of †P. contortus. Available illustrations furthermore support the assumption that †G. productus also is a junior synonym of †P. contortus.
Paleogene Tiger Sharks
Three species were not included in this analysis: one “interesting tooth” from the Eocene was described as a new species, †G. aeltrensis by Van Beneden (Reference Van Beneden and Van Bemmel1873: p. 385), but without illustrations or further information. Consequently, as descriptions of defining characters as well as illustrations are missing, we consider this species a nomen nudum. The second species, †G. priscus, is based solely upon some isolated scales from the late Eocene (Heckel Reference Heckel1853). Although isolated scales may bear paleoecological signals, they only provide very limited taxonomic information (Ferrón et al. Reference Ferrón, Pla, Martínez-Pérez, Escudero-Mozo and Botella2014), because different morphotypes occur across the body (e.g., Ankhelyi et al. Reference Ankhelyi, Wainwright and Lauder2018; Jambura and Kriwet Reference Jambura and Kriwet2020). Due to a lack of an illustration, it is not possible to unambiguously assign the described scales to a species, hence we consider †G. priscus a nomen dubium.
The third species, †G. aegyptiacus, was described by Stromer (Reference Stromer1905) based upon teeth previously assigned to †G. latidens by Dames (Reference Dames1883) and Stromer (Reference Stromer1903). Only very few records of †G. aegyptiacus are known (e.g., Stromer Reference Stromer1905; Underwood et al. Reference Underwood, Ward, King, Antar, Zalmout and Gingerich2011; Malyshkina et al. Reference Malyshkina, Gonzáles-Barba and Bannikov2013), and even these are considered doubtful (see Underwood et al. Reference Underwood, Ward, King, Antar, Zalmout and Gingerich2011; Sweydan et al. Reference Sweydan, Merzeraud, Essid, Marzougui, Temani, Ammar, Marivaux, Vianey-Liaud, Tabuce and Adnet2019). However, all specimens exhibit a cusp with a smooth distal cutting edge and a mesial cutting edge that is serrated along the lower two-thirds but smooth along the upper third, a character typically observed in the tiger shark–like genus †Physogaleus (Ebersole et al. Reference Ebersole, Cicimurri and Stringer2019). Recently, Ebersole et al. (Reference Ebersole, Cicimurri and Stringer2019) erected †P. alabamensis comb. nov. and included teeth formerly described as Galeocerdo, with the same tooth characteristics (e.g., mesial cutting edge serrated on the basis and smooth toward the apex, smooth distal cutting edge, slender and distally directed cusp, coarse serrations on distal heel diminishing in size basally) as seen in the few recorded teeth of †G. aegyptiacus; hence we propose to combine both species.
We analyzed teeth of the remaining Paleogene species †G. clarkensis, †G. eaglesomei, and the putative non-valid †G. latidens. The original description of †G. eaglesomei is based on the assumption of Dartevelle and Casier (Reference Dartevelle and Casier1943) that teeth figured as †G. latidens by White (Reference White1926) show distinct differences to the holotype of †G. latidens as described by Agassiz (Reference Agassiz1843). Nevertheless, Ebersole et al. (Reference Ebersole, Cicimurri and Stringer2019) synonymized †G. latidens with †G. eaglesomei, arguing that both constitute the same species, displaying a certain degree of monognathic heterodonty (i.e., morphological differences between anterior and lateral teeth), as also observed in the extant species, G. cuvier. Even though †G. latidens was described before †G. eaglesomei, Ebersole et al. (Reference Ebersole, Cicimurri and Stringer2019) decided to use the name †G. eaglesomei after merging both species, because the locality and horizon of the †G. latidens holotype are unknown, and therefore, it was considered a nomen dubium. Our results clearly support the merging of †G. eaglesomei and †G. latidens, as we could not detect significant differences between both species, which is further corroborated by a qualitative comparison of the tooth morphologies.
It is, however, noteworthy that †G. latidens appears to represent a wastebasket taxon for Eocene teeth over time; teeth originally described as †G. latidens have been assigned to other Galeocerdo species as well as to other genera, including Carcharhinus and †Physogaleus (see, e.g., Stromer Reference Stromer1905; White Reference White1955; Ebersole et al. Reference Ebersole, Cicimurri and Stringer2019). It therefore cannot be ruled out that “†G. latidens” includes more possibly unrecognized species.
†Galeocerdo clarkensis is an Eocene tiger shark species whose teeth differ from those of †G. eaglesomei in various aspects: the mesial cutting edge is evenly convex rather than sigmoidal (typical for anterior teeth of †G. eaglesomei), the serrations are compound (presence of secondary serrations on serrae) instead of being simple (Ebersole et al. Reference Ebersole, Cicimurri and Stringer2019), and the distal notch is more distinctly developed. This discrimination is also well supported by our results, where †G. clarkensis is well differentiated from †G. eaglesomei according to the pairwise comparison, emphasizing the validity of both species.
Neogene–Quaternary Tiger Sharks
The Neogene–Quaternary period (Miocene to Holocene) was initially characterized by a high diversity of tiger shark species. Of all species, three could not be included in the analysis: †G. similis, †G. sublaevis, and †G. pygmaeus from the Miocene were only mentioned but neither described nor illustrated (see Münster Reference Münster1842), making these species nomina nuda. Furthermore, our results indicate that †G. triqueter is a junior synonym of †P. contortus based on similar tooth morphologies and the same morphospace occupation (see “PCA on the Whole Sample”).
Within the analysis of the remaining Neogene–Quaternary tiger shark species, the most separated and distinct morphospace is the one occupied by †G. mayumbensis.
The Miocene species †G. bigelowi, †G. paulinoi, and †G. casei already were considered synonymous with †G. mayumbensis because of qualitative morphological similarities (Andrianavalona et al. Reference Andrianavalona, Ramihangihajason, Rasoamiaramanana, Ward, Ali and Samonds2015; Carrillo-Briceño et al. Reference Carrillo-Briceño, Luz, Hendy, Kocsis, Aguilera and Vennemann2019). In our study, †G. bigelowi and †G. paulinoi are both represented by one tooth each, and †G. casei by only two teeth. Hence, we were not able to provide robust statistical support for this assignment; however, all three species clearly share the same morphospace with †G. mayumbensis. The bulky tooth crowns, the strongly curved mesial cutting edges, and the Miocene age of all three species furthermore support synonymizing them with †G. mayumbensis.
In the current study, the Pliocene species †G. capellini is represented only by one tooth, and consequently, we cannot provide statistically informative results regarding this species. Teeth of †G. capellini are characterized by a large size and a distinct secondary serration (Lawley Reference Lawley1876). Purdy et al. (Reference Purdy, Schneider, Applegate, McLellan, Meyer and Slaughter2001) suggested merging †G. rosaliaensis with †G. capellini because of their similar morphology. Here, the examined teeth of both species plot within the same morphospace, supporting this merger. However, they are also largely overlapping with the extant G. cuvier and with †G. aduncus, which occurred until the late Miocene, possibly the early Pliocene. An assignment of both species to †G. aduncus can be ruled out, because teeth of †G. capellini and †G. rosaliaensis possess a distinct secondary serration (as also observed in G. cuvier) that separates them from the singly serrated †G. aduncus teeth. An assignment of †G. capellini and †G. rosaliaensis to G. cuvier was discussed before: Lawley (Reference Lawley1876) and Applegate (Reference Applegate1978) both emphasized the similarity of teeth of †G. capellini and †G. rosaliaensis, respectively, to the extant G. cuvier, and Cigala-Fulgosi and Mori (Reference Cigala-Fulgosi and Mori1979) eventually transferred †G. capellini to G. cuvier. However, Purdy et al. (Reference Purdy, Schneider, Applegate, McLellan, Meyer and Slaughter2001) proposed that a final decision must await a study on the dental variation of G. cuvier. In the present study, the morphospaces of †G. rosaliaensis and G. cuvier are highly overlapping, but nevertheless, both groups are statistically different. We therefore follow Purdy et al. (Reference Purdy, Schneider, Applegate, McLellan, Meyer and Slaughter2001) in considering †G. rosaliaensis a junior synonym of †G. capellini and keeping it separated from the extant tiger shark G. cuvier. However, we urge that a detailed reexamination of the holo- and syntypes of †G. capellini should be undertaken to clarify diagnostic characters for this species, because those described by Lawley (Reference Lawley1876) and Applegate (Reference Applegate1978) are not exclusive for †G. capellini. When photographing the specimens described by Applegate (Reference Applegate1978) for this study, one of the authors (F.A.L.-R.) noticed an extremely thick root compared with those of the extant G. cuvier, which could represent a useful character in species discrimination, pending future studies.
Due to the morphological similarity, Purdy et al. (Reference Purdy, Schneider, Applegate, McLellan, Meyer and Slaughter2001) suggested that teeth of †G. aduncus represent juvenile teeth of the extant G. cuvier. Traditionally, †G. aduncus is separated from G. cuvier based on the size as well as the absence of secondary serrations on the mesial cutting edge (Stoutamire Reference Stoutamire1975; Applegate Reference Applegate1978; Cigala-Fulgosi and Mori Reference Cigala-Fulgosi and Mori1979; Kent Reference Kent2018). The present geometric morphometric analysis depicts a strong overlap of †G. aduncus and G. cuvier teeth, especially along the first PC axis, which describes the monognathic heterodonty. However, both species are perceivably more separated along PC 2 (describing shape differences of the root lobe and the breadth of the crown) and are clearly different species according to the ANOVA. These results, in addition to the absence of a secondarily serrated mesial cutting edge, clearly demonstrate that †G. aduncus has to be considered a valid species.
A secondary serration is also lacking in teeth of †G. davisi and †G. gajensis, and furthermore, both species plot within the three overlapping morphospaces of †G. aduncus, G. cuvier, and †G. rosaliaensis. Although both taxa are represented by only one tooth each, which complicates the interpretation of the results, as no statistical clarity is present, the similar tooth morphology and the simple serration clearly indicate that †G. davisi and †G. gajensis should be considered synonymous with †G. aduncus.
A distinct dignathic heterodonty (i.e., morphological differences between teeth of upper and lower jaws) is known from several carcharhiniform sharks (Compagno Reference Compagno1988), with the extant tiger shark G. cuvier being very exceptional, as the teeth of both jaws are very similar in appearance. A lack of an articulated dentition of any fossil tiger shark species complicates the question of whether extinct Galeocerdo species already possessed a monognathic dentition or not. Notably, the dentition of †G. aduncus was at the center of intensive discussions regarding possible heterodonties for a long time, and different authors provided various hypotheses, including that a well-developed dignathic heterodonty occurred in †G. aduncus, with †P. contortus–type teeth in the lower jaw and typical cockscomb-shaped teeth in the upper jaw (Applegate Reference Applegate1978, Reference Applegate1992), or that †G. aduncus displays a gynandric heterodonty, with only males having slender tooth crowns (Ward and Bonavia Reference Ward and Bonavia2001). Ward and Bonavia (Reference Ward and Bonavia2001) accordingly assumed that teeth of †P. contortus represent teeth of male †G. aduncus and merged both species into †P. aduncus.
The main reason for rejecting the hypothesis of a distinct dignathic heterodonty with †G. aduncus–type teeth in the upper jaw and †P. contortus–type teeth in the lower jaw of †G. aduncus is the fact that there are localities where only teeth with either one of these two morphologies occur (Kent Reference Kent2018). A possible gynandric heterodonty, however, would indicate a strong segregation by sex at the respective localities (Reinecke et al. Reference Reinecke, Louwye, Havekost and Moths2011). In the extant tiger shark, sexual separation is only known to occur seasonally at some sites, for example, Tiger Beach in the western central Atlantic (Bahamas), where mostly female sharks of different life stages reside to reach maturity with less male mating harassment and to use the warm environment to reduce the gestation periods (Lea et al. Reference Lea, Wetherbee, Queiroz, Burnie, Aming, Sousa, Mucientes, Humphries, Harvey, Sims and Shivji2015; Sulikowski et al. Reference Sulikowski, Wheeler, Gallagher, Prohaska, Langan and Hammerschlag2016).
Kent (Reference Kent2018) supported the hypothesis of †G. aduncus and †P. contortus being separate species but nevertheless assumed a certain heterodonty in †G. aduncus. He described three types of tooth morphologies for both species, comprising a broad and a narrow morphology in †G. aduncus and teeth with the typical †P. contortus morphology. The dataset of †G. aduncus in the present study contained teeth with broad and narrow morphologies, both clustering together. However, both types of teeth together showed distinct differences to teeth unambiguously representing †P. contortus (Fig. 8). This demonstrates the presence of a heterodont dentition in †G. aduncus, with broad and narrow teeth, which nevertheless are clearly different from †P. contortus, and therefore the validity of both taxa. Although a certain heterodonty, either dignathic or gynandric, seemingly exists in †G. aduncus, it is not to the extent previously proposed (i.e., including †P. contortus–type teeth). The presence of broad and narrow tooth morphologies in †G. aduncus is furthermore a character that differentiates its teeth from those of G. cuvier and hence can be used to distinguish both species.
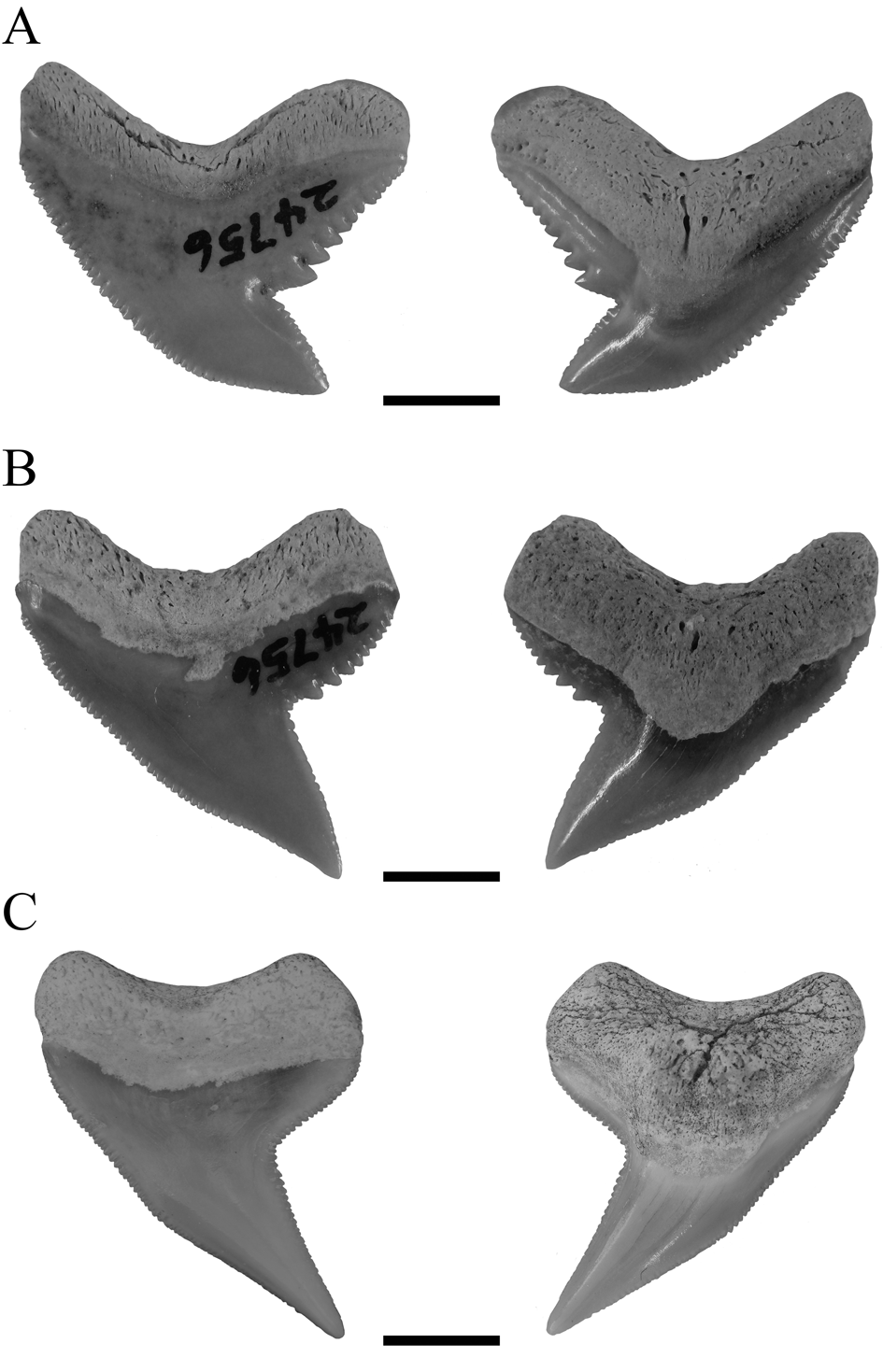
Figure 8. Isolated fossil teeth of †G. aduncus and †P. contortus in labial and lingual positions. A, Broad tooth morphology of †G. aduncus. B, Narrow tooth morphology of †G. aduncus. C, Typical tooth morphology of †P. contortus. Scale bars, 5 mm.
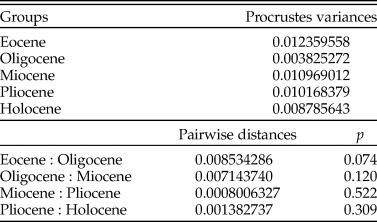
Typically, it is assumed that the sole extant tiger shark G. cuvier evolved in the late Miocene/early Pliocene. However, dos Reis (Reference dos Reis2005), Pimiento et al. (Reference Pimiento, Gonzales-Barba, Hendy, Jaramillo, MacFadden, Montes, Suarez and Shippritt2013), and Patnaik et al. (Reference Patnaik, Sharma, Mohan, Williams, Kay and Chatrath2014) indicated the presence of G. cuvier in the early Miocene. The tooth described by dos Reis (Reference dos Reis2005) clearly belongs to †G. mayumbensis, as demonstrated by our analyses here (Fig. 6), also indicated by Carrillo-Briceño et al. (Reference Carrillo-Briceño, Luz, Hendy, Kocsis, Aguilera and Vennemann2019). The teeth described by Pimiento et al. (Reference Pimiento, Gonzales-Barba, Hendy, Jaramillo, MacFadden, Montes, Suarez and Shippritt2013) and Patnaik et al. (Reference Patnaik, Sharma, Mohan, Williams, Kay and Chatrath2014) as G. cuvier were not included in this study but already were demonstrated to belong to †G. mayumbensis by Carrillo-Briceño et al. (Reference Carrillo-Briceño, Luz, Hendy, Kocsis, Aguilera and Vennemann2019), an interpretation with which we agree. So far, no unambiguous record of G. cuvier from the early Miocene has been reported. However, 37 teeth from middle Miocene deposits of Florida, USA (Supplementary Table 2), are identified unambiguously as those of G. cuvier here, based on distinct diagnostic characters for G. cuvier teeth, such as the presence of secondary serrations (Fig. 9). These records extend the origin of the extant species from the late Miocene/early Pliocene (ca. 5.3 Ma) back into the middle Miocene (ca. 13.8 Ma).
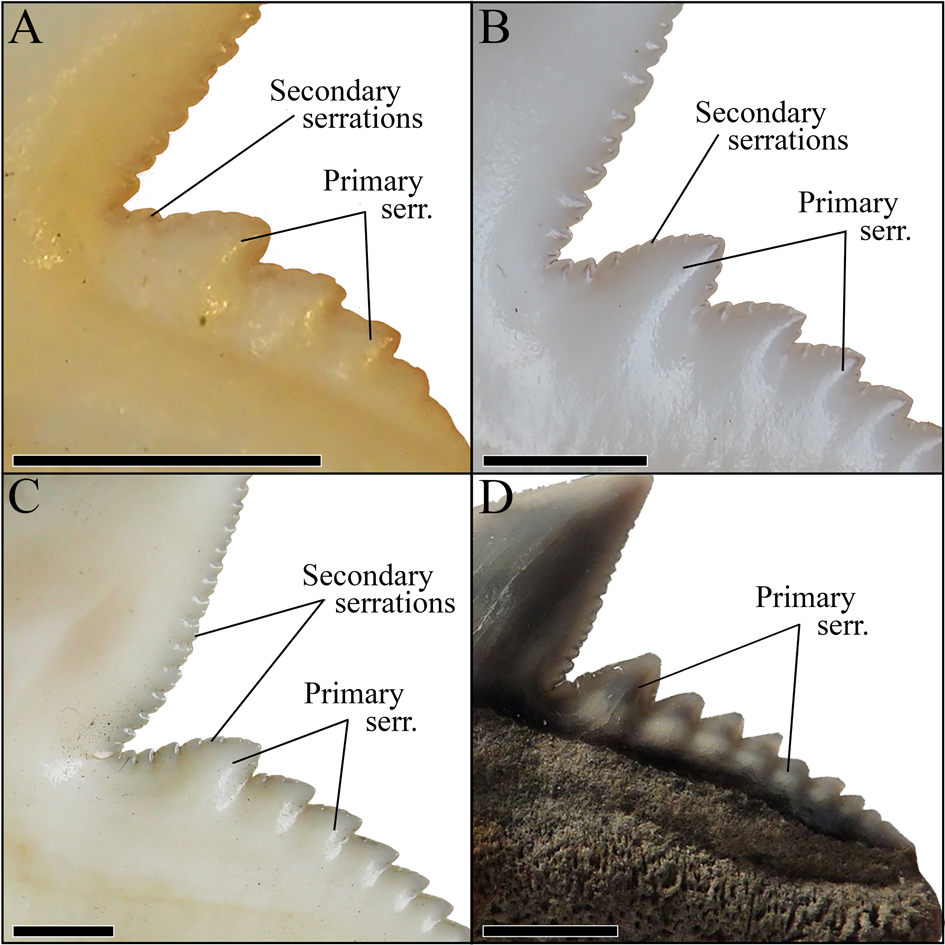
Figure 9. Magnification of the distal cutting edges and distal heels of the teeth of three different G. cuvier specimens and one †G. aduncus specimen, identifying the primary and secondary serrations. A, Anterior tooth of a juvenile G. cuvier specimen. B, Anterior tooth of a subadult G. cuvier specimen. C, Lateral tooth of an adult G. cuvier specimen. D, Isolated lateral tooth of †G. aduncus. Scale bars, 2 mm.
Disparity Patterns through Time
When the number of tiger shark species observed in this study is compared to the dental disparity of Galeocerdo through time, the most conspicuous feature is the contrasting development of both. Although only two validated taxa of tiger sharks were present throughout the Eocene (†G. clarkensis and †G. eaglesomei), the disparity of the dental morphology attained its highest levels during this time. Conversely, four species are present throughout the Neogene to Quaternary (†G. aduncus, †G. capellini, G. cuvier, †G. mayumbensis), but the dental disparity was lower than in the Eocene. Another striking feature is the extremely high disparity from the middle Miocene to today, although the species diversity decreased even further to a single extant species. However, patterns with the highest disparity attained early in the evolution were likewise observed for several other clades (e.g., Erwin Reference Erwin2007; Hughes et al. Reference Hughes, Gerber and Wills2013).
In contrast to †G. eaglesomei, †G. clarkensis already developed the typical cockscomb-shaped dentition, resembling that of the extant G. cuvier. Hence, the teeth of †G. eaglesomei, characterized by the weakly developed distal notch distinguishing it from all other tiger shark species, are especially accountable for the high disparity in the Eocene. The Eocene/Oligocene transition was a period of substantial extinction and global ecological change due to decreasing sea temperatures and ice sheet formation on Antarctica (e.g., Coxall and Pearson Reference Coxall, Pearson, Williams, Haywood, Gregory and Schmidt2007; Goldner et al. Reference Goldner, Herold and Huber2014). During this time, the dental disparity of tiger sharks dropped to its lowest level, congruent with the extinction of †G. clarkensis and †G. eaglesomei and the origination of †G. aduncus. However, the low number of Oligocene specimens included in this study (n = 3) does not allow statistically significant conclusions, and the possibility of a higher dental disparity of tiger sharks cannot be excluded. Throughout the Neogene and Quaternary, the diversity of tiger sharks increased to four species (†G. aduncus, †G. capellini, G. cuvier, †G. mayumbensis). Both †G. aduncus and †G. mayumbensis vanished in the late Miocene/early Pliocene, and †G. capellini is only known from Pliocene deposits so far, resulting in only a single extant species, G. cuvier. Instead of dropping considerably with the taxonomic diversity, the dental disparity decreased only sparsely and remained at a high level from the middle Miocene until today, a pattern attributed to the highly similar tooth morphologies of the Neogene–Quaternary species, especially of †G. aduncus, †G. capellini, and G. cuvier. A similar pattern was observed across the Cretaceous/Paleogene mass extinction: although 84% of all shark species were lost during this event (Kriwet and Benton Reference Kriwet and Benton2004), the dental disparity of lamniform and carcharhiniform sharks stayed nearly static (Bazzi et al. Reference Bazzi, Kear, Blom, Ahlberg and Campione2018).
Conclusions
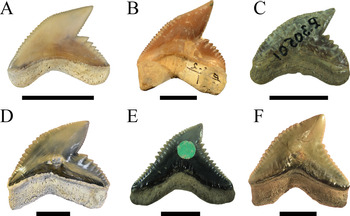
We demonstrate here that the presumed high taxonomic diversity of extinct tiger sharks, compared with only one extant species, was much lower than generally assumed. Applying multivariate analyses, we were able to successfully distinguish between six tiger shark species from the Eocene to Holocene instead of 23 (Figs. 1, 10). These species comprise the Eocene †G. clarkensis and †G. eaglesomei, the Oligocene to late Miocene †G. aduncus, the Miocene †G. mayumbensis, the Pliocene †G. capellini, and the extant tiger shark G. cuvier, with a range extension back into the middle Miocene. Our results corroborate that the combined approach of quantitative geometric morphometric techniques and qualitative morphological comparisons is appropriate to identify taxa known only by isolated teeth and to differentiate between teeth of extinct and extant species despite high morphological resemblances. The new analyses moreover show that declining diversity and disparity are not implicitly correlated, which is revealed by the high dental disparity of Galeocerdo since the Miocene despite the decrease from four to only one species. Further studies on the dental disparity in sharks in deep time are mandatory to enhance our understanding of extinction patterns and the inherent link between taxonomic diversity and morphological disparity in this group of marine apex predators.
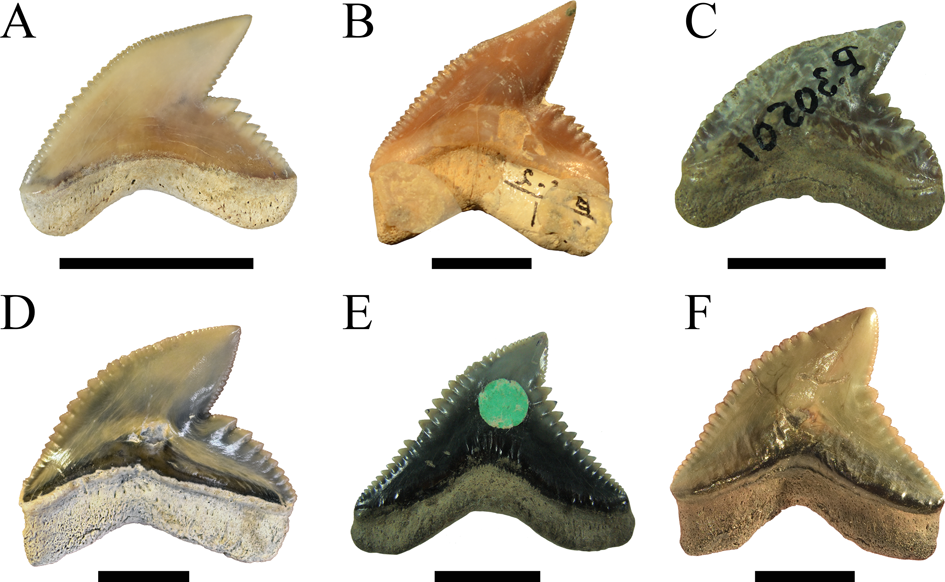
Figure 10. Isolated fossil teeth of the six valid tiger shark species. A, †Galeocerdo aduncus. B, †Galeocerdo capellini. C, †Galeocerdo clarkensis holotype. D, Galeocerdo cuvier, E, †Galeocerdo eaglesomei holotype. F, †Galeocerdo mayumbensis. Scale bars, 10 mm.
Acknowledgments
We are grateful to the following people for their help with access and for granting permissions to study material in their collections: E. Bernard (Natural History Museum London), U. Göhlich (Natural History Museum Vienna), B. MacFadden (Florida Museum of Natural History), A. Millhouse (Smithsonian National Museum of Natural History), T. Mörs (Swedish Museum of Natural History), J. Alvarado-Ortega and V. A. Romero Mayén (Colección Nacional de Paleontología, Universidad Nacional Autónoma de México). We also want to thank C. J. Underwood and one anonymous reviewer for their constructive feedback on an earlier version of the article and N. Froebisch for editorial comments. This study was supported by a grant of the Austrian Science Fund (FWF): P 33820 to J.K.
Data Availability Statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.rv15dv47c.
Supplementary Material: Morphological descriptions of the 23 previously recognized extinct and extant tiger shark species.
Supplementary Table 1: Summary of all examined shark teeth of the geometric morphometric study.
Supplementary Table 2: List of all examined middle Miocene G. cuvier teeth.