Published online by Cambridge University Press: 20 May 2022
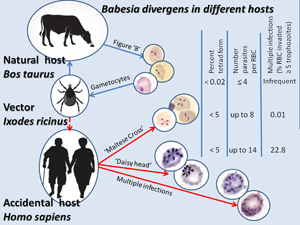
Babesias are obligate apicomplexan parasites that affect the red blood cells (RBCs) of animals. Humans can serve as accidental hosts for them. Asexual reproduction of a parasite occurs in a vertebrate host through asynchronous binary fission, yielding a complex pleomorphic population of intraerythrocytic forms. In natural hosts (Bos taurus), paired pyriforms (‘figure 8’) of Babesia divergens are usual, but tetrads (‘Maltese Cross’) are very rare (only in 0.02% infected erythrocytes); in humans, however, up to 5% of infected erythrocytes show tetrads. The current study shows that B. divergens proliferating in an accidental human host can promote extraordinarily high level of fission. This phenomenon is expressed as the simultaneous division of the parasite into 6 and possibly a greater number of merozoites, forming a ‘daisy head’ (vs the usual 2, less often 4 merozoites). Reproduction is possible without egressing merozoites from the erythrocyte, which results in multi-occupancy of an RBC (≥5 parasites per RBC). An unusually high polyparasitism – up to 14 parasites developed in the affected erythrocytes – was observed. This phenomenon is rare in natural hosts (usually ≤5), but when B. divergens is cultured in vitro it can be 10–12.