Introduction
The snail-borne neglected tropical disease (NTD), schistosomiasis, is the most important freshwater parasitic disease of humans associated with poverty, poor sanitation and lack of safe water supplies (Steinmann et al., Reference Steinmann, Keiser, Bos, Tanner and Utzinger2006; Hotez et al., Reference Hotez, Alvarado, Basáñez, Bolliger, Bourne, Boussinesq, Brooker, Brown, Buckle, Budke, Carabin, Coffeng, Fèvre, Fürst, Halasa, Jasrasaria, Johns, Keiser, King, Lozano, Murdoch, O'Hanlon, Pion, Pullan, Ramaiah, Roberts, Shepard, Smith, Stolk, Undurraga, Utzinger, Wang, Murray and Naghavi2014), with an estimated 180–200 million people primarily from low- and middle-income countries being infected (GBD 2016 Disease and Injury Incidence and Prevalence Collaborators, 2017). Ambitious goals to eliminate schistosomiasis have been announced by the WHO as part of its roadmap to overcome the global impact of NTDs by 2020–2025 (WHO, 2012). Whilst mass drug administration, behavioural change through education and snail control are having a major impact on schistosomiasis, further research into schistosome transmission biology together with better tools for transmission monitoring and surveillance are required to help achieve and monitor the success of these ambitious goals (Stothard et al., Reference Stothard, Campbell, Osei-Atweneboana, Durant, Stanton, Biritwum, Rollinson, Ombede and Tchuem-Tchuenté2017). Schistosomiasis is also a disease of animals, with large numbers of domestic livestock affected worldwide but the actual veterinary and economic impact is largely unknown (De Bont and Vercruysse, Reference De Bont and Vercruysse1997, Reference De Bont and Vercruysse1998).
There are 25 recognized species of mammalian schistosomes that cause human and animal infections, which can be split into four Schistosoma species groups (Webster et al., Reference Webster, Southgate and Littlewood2006). The largest group is the Schistosoma haematobium group containing nine species that are all transmitted through Bulinus snails (Brown, Reference Brown1994) with two species, S. haematobium and S. bovis, being responsible for the majority of all human (Hotez and Kamath, Reference Hotez and Kamath2009) and livestock infections (De Bont and Vercruysse, Reference De Bont and Vercruysse1997), respectively. Central to this group is S. haematobium, a major human schistosome species being the most widespread and prevalent across Africa and solely responsible for human urogenital schistosomiasis with often severe pathology (Schwartz, Reference Schwartz1981; Leutscher et al., Reference Leutscher, Ramarokoto, Reimert, Feldmeier, Esterre and Vennervald2000; Bustinduy et al., Reference Bustinduy, King, Scott, Appleton, Sousa-Figueiredo, Betson and Stothard2014; Kjetland et al., Reference Kjetland, Hegertun, Baay, Onsrud, Ndhlovu and Taylor2014; Christinet et al., Reference Christinet, Lazdins-Helds, Stothard and Reinhard-Rupp2016). Schistosoma bovis is a pathogen of domestic livestock and some artiodactylids (Standley et al., Reference Standley, Mugisha, Dobson and Stothard2012), with its distribution commonly overlapping with that of S. haematobium across mainland Africa (Moné et al., Reference Moné, Mouahid and Morand1999), and utilising a wide range of Bulinus (Southgate and Knowles, Reference Southgate and Knowles1975a, Reference Southgate and Knowles1975b; Stothard et al., Reference Stothard, Lockyer, Kabatereine, Tukahebwa, Kazibwe, Rollinson and Fenwick2004). These two species, among others, are also able to hybridize and inter-specific hybridization is now recognized in West Africa with possible detrimental consequences on disease control (Huyse et al., Reference Huyse, Webster, Geldof, Stothard, Diaw, Polman and Rollinson2009; Webster et al., Reference Webster, Diaw, Seye, Webster and Rollinson2013; Léger and Webster, Reference Léger and Webster2017).
Pemba and Unguja Islands (Zanzibar Archipelago, United Republic of Tanzania) have been historically identified as ‘model islands’ for implementing multiple effective infectious disease control and elimination programmes in sub-Saharan Africa (Pennance et al., Reference Pennance, Person, Muhsin, Khamis, Muhsin, Khamis, Mohammed, Kabole, Rollinson and Knopp2016). For schistosomiasis control, Zanzibar also offers an advantage due to the allopatric transmission of S. haematobium through a single snail host, Bulinus globosus, on both Islands (Stothard et al., Reference Stothard, Loxton, Rollinson, Mgeni, Khamis, Ameri, Ramsan and Savioli2000), whereas across most of sub-Saharan Africa, multiple Schistosoma and Bulinus species occur in sympatry (Brown, Reference Brown1994), complicating control interventions and surveillance. Urogenital schistosomiasis was highly endemic on both islands but is now targeted for elimination (Knopp et al., Reference Knopp, Mohammed, Ali, Khamis, Ame, Albonico, Gouvras, Fenwick, Savioli and Colley2012, Reference Knopp, Person, Ame, Mohammed, Ali, Khamis, Rabone, Allan, Gouvras and Blair2013).
As we move towards or reach elimination, there becomes a need for more sensitive methods to monitor the levels of transmission when egg–patent human infections become scarce (Le and Hsieh, Reference Le and Hsieh2017; Stothard et al., Reference Stothard, Campbell, Osei-Atweneboana, Durant, Stanton, Biritwum, Rollinson, Ombede and Tchuem-Tchuenté2017), the risk of infection and also a way to prove transmission interruption when it is finally reached. Xenomonitoring is a nucleic acid-based molecular diagnostic used to monitor the transmission of several vector-borne diseases (Cunningham et al., Reference Cunningham, Lingley, Haines, Ndung'u, Torr and Adams2016; Minetti et al., Reference Minetti, LaCourse, Reimer and Storhard2016; Cook et al., Reference Cook, Pilotte, Minetti, Williams and Reimer2017), including to some extent schistosomiasis where tools are being developed for the xenomonitoring of snails that could support schistosomiasis transmission and elimination monitoring (Hamburger et al., Reference Hamburger, Hoffman, Kariuki, Muchiri, Ouma, Koech, Sturrock and King2004; Allan et al., Reference Allan, Dunn, Emery, Stothard, Johnston, Kane, Khamis, Mohammed and Rollinson2013; Lu et al., Reference Lu, Zhang, Mutuku, Mkoji and Loker2016; Abbasi et al., Reference Abbasi, Webster, King, Rollinson and Hamburger2017). The first stage for snail xenomonitoring for schistosomiasis is the identification of patent schistosome infections within the snails and collecting cercariae shed from them. Here, we report on the molecular identification of these cercariae and the infected snails collected from Pemba Island (Zanzibar) and how the findings complicate the development of robust molecular xenomonitoring protocols for ongoing and future transmission monitoring.
Methods
Malacological surveys and Schistosoma collection
In November 2016, as part of a larger ongoing molecular xenomonitoring study on Pemba, Bulinus snails were collected, by scooping, from human freshwater contact sites in eight shehias (smallest division of administrative regions), examined and individually induced to shed cercariae following previous methods (Allan et al., Reference Allan, Dunn, Emery, Stothard, Johnston, Kane, Khamis, Mohammed and Rollinson2013). An experienced microscopist identified schistosome cercariae, which were individually pipetted in 3.5 µL aliquots onto Whatman FTA cards (Whatman, Part of GE Healthcare, Florham Park, USA) for long-term deoxyribonucleic acid (DNA) storage. After shedding, all infected snails were preserved in 100% ethanol for future morphological and molecular characterization.
Schistosoma and Bulinus identification
DNA from individual cercariae was eluted from the FTA cards (Webster et al., Reference Webster, Rabone, Pennance, Emery, Allan, Gouvras, Knopp, Garba, Hamidou, Mohammed, Ame, Rollinson and Webster2015) and characterized by amplification and sequencing of the mitochondrial cytochrome oxidase subunit 1 (cox1) and partial nuclear internal transcribed spacer (ITS1 + 2) DNA regions (Webster et al., Reference Webster, Emery, Webster, Gouvras, Garba, Diaw, Seye, Tchuente, Simoonga, Mwanga, Lange, Kariuki, Mohammed, Stothard and Rollinson2012).
To determine the species of the infected snails, total genomic DNA was extracted from the whole snail tissue using the DNeasy Blood & Tissue Kit (Qiagen, Manchester, UK), with minor changes to the standard protocol in that quantities of the digest reagents were doubled and digests were incubated for at least 12 h. From each snail, a 623 base pair region of the mitochondrial cox1 gene was amplified and Sanger sequenced using primers BulCox1 and CO2 following previous protocols (Kane et al., Reference Kane, Stothard, Emery and Rollinson2008). The sequence data were manually edited in Sequencher v5.1 (http://genecodes.com) before being compared with reference sequence databases for Bulinus (Kane et al., Reference Kane, Stothard, Emery and Rollinson2008) and Schistosoma (Webster et al., Reference Webster, Emery, Webster, Gouvras, Garba, Diaw, Seye, Tchuente, Simoonga, Mwanga, Lange, Kariuki, Mohammed, Stothard and Rollinson2012, Reference Webster, Diaw, Seye, Webster and Rollinson2013) to confirm species.
Results
In total, 1317 B. globosus and B. nasutus were collected, seven of these snails (Table 1) from Kinyasini (6) and Chambani (1) shehia were shedding schistosome cercariae (Fig. 1). The infected snails were identified as B. globosus with two cox1 haplotypes recognized (GenBank accession numbers: MH014040 and MH014041) which matched those snails previously reported from Pemba (Kane et al., Reference Kane, Stothard, Emery and Rollinson2008). Cercariae collected from these were assumed initially to be the human parasite S. haematobium; however, molecular characterizations of the cercariae from five of these snails, collected from a stream in Kinyasini (Kinya6), were identified as S. bovis (Table 1). Two different S. bovis cox1 haplotypes [Genbank accessions: S.b (i) MH014042 and S.b (ii) MH014043] (Table 1) were identified from these five snails; three snails producing S. bovis cercariae of a single haplotype and two snails producing S. bovis cercariae of both haplotypes suggesting that they had been infected by more than one miracidium.
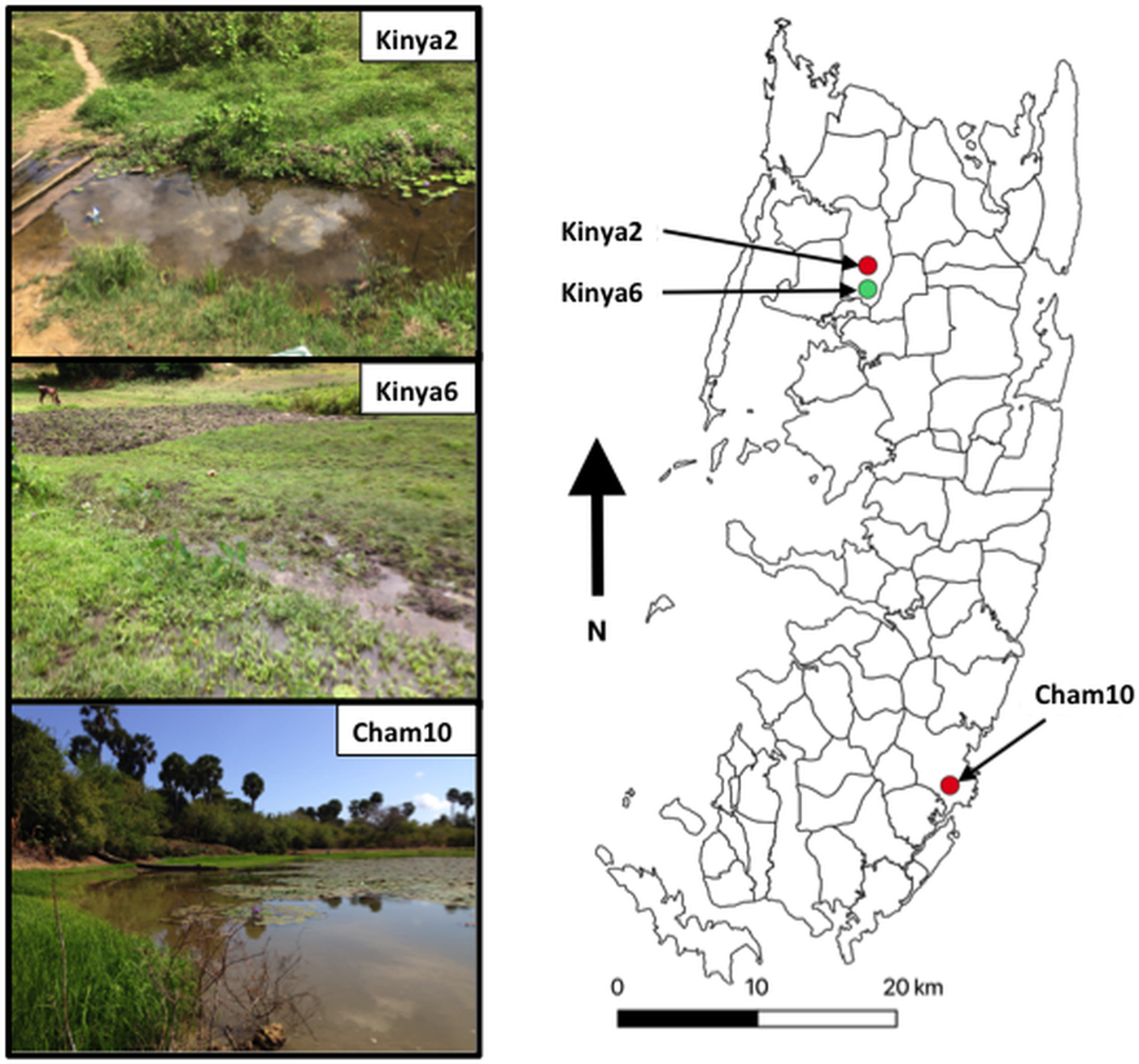
Fig. 1. Map outlining shehias (smallest division of administrative regions) on Pemba Island, Zanzibar (United Republic of Tanzania) showing the location and images of two freshwater bodies in Kinyasini (Kinya2 and Kinya6) and one in Chambani (Cham10) where Schistosoma haematobium (red) and Schistosoma bovis (green) cercariae were recovered from Bulinus globosus. GPS coordinates for sites (latitude and longitude in decimal degrees): Kinya2 (−5.02033°, 39.73855°); Kinya6 (−5.03560°, 39.73850°); Cham10 (−5.35805°, 39.79182°).
Table 1. Showing the collection sites and genetic profiles of the Bulinus and schistosome cercariae analysed
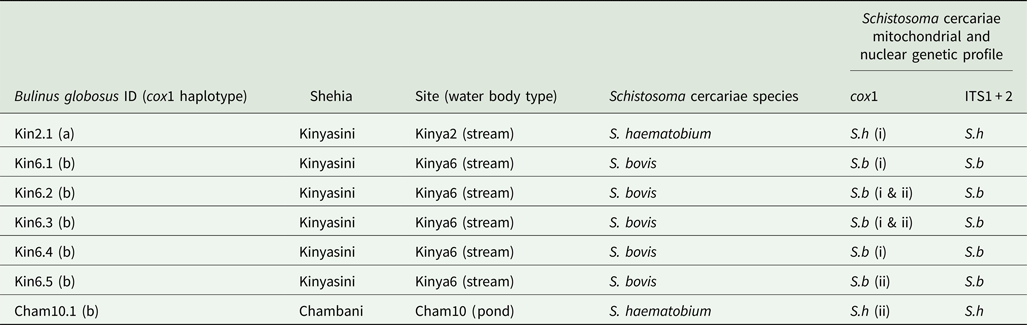
Two Bulinus globosus cox1 haplotypes [Genbank accessions: (a) MH014040 and (b) MH014041]. Two S. haematobium cercariae cox1 haplotypes, Genbank accessions: S.h (i) MH014046 and S.h (ii) MH01404 and the two S. bovis cox1 haplotypes, Genbank accessions: S.b (i) MH014042 and S.b (ii) MH014043. ITS1 + 2 profiles showed no intra species variation (Genbank accessions: S.h MH014047 and S.b MH014044).
The other two infected snails shed S. haematobium cercariae and were collected from a pond in Chambani (Cham10) and a different stream site in Kinyasini (Kinya2). The S. haematobium cercariae from Kinyasini and Chambani, respectively, were of two different S. haematobium cox1 haplotypes [Genbank accessions: S.h (i) MH014046 and S.h (ii) MH014045] with only single haplotypes produced from each snail. These haplotypes matched those identified as group 2 S. haematobium cox1 haplotypes found only in the Indian Ocean Islands (Webster et al., Reference Webster, Emery, Webster, Gouvras, Garba, Diaw, Seye, Tchuente, Simoonga, Mwanga, Lange, Kariuki, Mohammed, Stothard and Rollinson2012).
ITS1 + 2 profiles showed no intra-species variation (Genbank Accessions: S.h MH014047 and S.b MH014044) and were identified as either S. bovis or S. haematobium by the three inter-specific single nucleotide polymorphisms (Webster et al., Reference Webster, Emery, Webster, Gouvras, Garba, Diaw, Seye, Tchuente, Simoonga, Mwanga, Lange, Kariuki, Mohammed, Stothard and Rollinson2012).
Discussion
The detection of S. bovis on Pemba Island poses a potentially new threat to domestic livestock and wildlife health in Zanzibar (De Bont and Vercruysse, Reference De Bont and Vercruysse1997, Reference De Bont and Vercruysse1998; Standley et al., Reference Standley, Mugisha, Dobson and Stothard2012). The site where S. bovis transmission was identified had grazing cattle (see Fig. 1, Kinya6) in close proximity to the water where the shedding snails were collected; therefore, it is quite likely that ongoing transmission is being maintained. Moreover, the movement of infected cattle could enable the spread of the infection particularly as B. globosus are found throughout most of the island (Stothard et al., Reference Stothard, Mgeni, Alawi, Savioli and Rollinson1997).
The presence of S. bovis complicates the monitoring of S. haematobium transmission since both parasites are shown here to infect the same intermediate snail host and cannot be distinguished from each other easily by microscopy. Therefore, S. bovis-infected B. globosus could be falsely identified as infected with S. haematobium, or vice-versa, complicating urogenital schistosomiasis transmission monitoring. This accentuates the need for routine molecular identification of schistosome infections in snails during malacological surveys (Minetti et al., Reference Minetti, LaCourse, Reimer and Storhard2016), and the development of more species-specific xenomonitoring tools to differentiate S. bovis and S. haematobium transmission (Webster et al., Reference Webster, Rollinson, Stothard and Huyse2010; Abbasi et al., Reference Abbasi, Webster, King, Rollinson and Hamburger2017). The identification of schistosome cercariae shed from snails is often presumed to be of a particular species due to the snail host involved or the locality of the transmission. Our findings strongly emphasize that these assumptions are not accurate and transmission dynamics of different species may change over time and space. The assumed transmission of only S. haematobium by B. globosus on Zanzibar and the non-identification of these S. bovis infections would have led us to believe that the level of S. haematobium transmission is much higher than it actually is, hampering ongoing and future urogenital schistosomiasis transmission monitoring and surveillance.
Schistosoma haematobium and S. bovis hybridization has also been detected in sympatric West African areas (Webster et al., Reference Webster, Diaw, Seye, Webster and Rollinson2013). Zanzibar was considered to be an allopatric area for S. haematobium (Webster et al., Reference Webster, Emery, Webster, Gouvras, Garba, Diaw, Seye, Tchuente, Simoonga, Mwanga, Lange, Kariuki, Mohammed, Stothard and Rollinson2012) but the identification of this sympatry with S. bovis could, in time, lead to inter-species hybridization. The potential consequences of hybridization include increased host associations of hybrids, possible zoonotic transmission and hybrid vigour (Huyse et al., Reference Huyse, Webster, Geldof, Stothard, Diaw, Polman and Rollinson2009; Webster et al., Reference Webster, Diaw, Seye, Webster and Rollinson2013; Léger and Webster, Reference Léger and Webster2017). Investigating the origin of S. bovis being transmitted on Pemba, by genetic comparison with other mainland strains of S. bovis, may help elucidate how this parasite has been imported to Zanzibar. Since the eradication of the tsetse fly, the vector of human and African animal trypanosomiasis, on Unguja Island (Vreysen et al., Reference Vreysen, Saleh, Ali, Abdulla, Zhu, Juma, Dyck, Msangi, Mkonyi and Feldmann2000), there has been an increase of cattle farming (Mdoe, Reference Mdoe2003) facilitated by the import of cattle under strict guidelines of the United Republic of Tanzania's Animal Resources Management Act (1999). Bovine schistosomiasis however is widely ignored/unknown as a veterinary health problem, and therefore is currently not included in these guidelines. This oversight could offer some explanation to how and within what time scale the introduction, or multiple introductions, of S. bovis may have occurred. Additionally, the prevalence and intensity of S. bovis in local cattle and other potential artiodactylid hosts (Standley et al., Reference Standley, Mugisha, Dobson and Stothard2012), such as the Ader's duiker (Cephalophus adersi) endemic to Zanzibar, should be determined to assess the impact on livestock and wildlife health. However, diagnosing S. bovis from the definitive host remains challenging, with the detection of S. bovis eggs in the stool being difficult and the more sensitive method of observing adult worms in the host being only possible post-mortem via dissection. An antigen-based test with promising diagnostic performance has been developed (de la Torre-Escudero et al., Reference de la Torre-Escudero, Manzano-Román, Pérez-Sánchez, Barrera, Siles-Lucas and Oleaga2012), which could offer a sensitive method for judging the epidemiology of S. bovis in Pemba.
Due to the difficulty in classifying species within the Bulinus africanus species complex (Kane et al., Reference Kane, Stothard, Emery and Rollinson2008), previous findings on snail–schistosome compatibilities should be treated with some caution. This molecular confirmation of B. globosus naturally transmitting S. bovis in East Africa gives credibility to a previous observation (Mwambungu, Reference Mwambungu1988), and dispels previous claims of B. globosus being naturally refractory (Christensen et al., Reference Christensen, Mutani and Frandsen1983) or only an intermediate host in West Africa (Diaw and Vassiliades, Reference Diaw and Vassiliades1987; Ndifon et al., Reference Ndifon, Betterton and Rollinson1988). Previous evidence for compatibility of B. nasutus with S. bovis in East Africa is also tainted with contradicting evidence, some showing natural infections (Dowdeswell, Reference Dowdeswell1938; Kinoti, Reference Kinoti1964b) going against failed experimental infections (Southgate and Knowles, Reference Southgate and Knowles1975a, Reference Southgate and Knowles1975b; Southgate et al., Reference Southgate, Rollinson, Ross and Knowles1980) and a lack of naturally infected B. nasutus in other endemic areas (Kinoti, Reference Kinoti1964a; Southgate et al., Reference Southgate, Rollinson, Ross and Knowles1980; Mutani et al., Reference Mutani, Christensen and Frandsen1983). It is likely that S. bovis has a broad intermediate host range in East Africa utilising several Bulinus species, as it has also been identified from B. ugandae (Malek, Reference Malek1969), B. africanus (McClelland, Reference McClelland1955; Teesdale and Nelson, Reference Teesdale and Nelson1958; Kassuku et al., Reference Kassuku, Christensen, Monrad, Nansen and Knudsen1986) and B. forskalii (McClelland, Reference McClelland1955). Therefore, studies to confirm the intermediate snail host vectoral capacity and specificity of S. bovis to B. globosus or indeed other endemic Bulinus species on Pemba, including B. nasutus and B. forskalii, are required to determine the transmission potential and possible spread of this emerging schistosome in Zanzibar.
Acknowledgements
Thanks to Said Mohammed Ali and staff at the Public Health Laboratory – Ivo de Carneri for making the surveys and collections possible, and also to Dr Steffi Knopp at the Swiss Tropical and Public Health Institute in Basel for helping to identify study sites. Thanks also to the Natural History Museums DNA Sequencing Facilities for the sequencing services.
Financial support
The authors would like to thank the London Centre for Neglected Tropical Disease Research (LCNTDR) and University of Georgia Research Foundation, Inc., which was funded by the Bill & Melinda Gates Foundation for the Schistosomiasis Consortium for Operational Research and Evaluation (SCORE) project, for funding TP and BW, respectively, for travel and expenses to undertake the field collections in November 2016. FA is financially supported by the Wellcome Trust (SCAN Project WT104958MA). The authors would also like to acknowledge the Natural History Museum's Departmental Investment Fund for the financial support facilitating the molecular work.
Conflict of interest
None.
Ethical standards
Not applicable.





