INTRODUCTION
In the past three decades, Echinococcus multilocularis, the causative agent of human alveolar echinococcosis (AE), has been newly reported in countries of Europe (Davidson et al. Reference Davidson, Romig, Jenkins, Tryland and Robertson2012) and discovered in foci of relatively high incidence in China and neighbouring countries of Central Asia and Siberia (Craig et al. Reference Craig, Liu, Macpherson, Shi, Reynolds, Barnish, Gottstein and Wang1992; Ito et al. Reference Ito, Urbani, Qiu, Vuitton, Qiu, Heath, Craig, Zheng and Schantz2003, Reference Ito, Agvaandaram, Bat-Ochir, Chuluunbaatar, Gonchingsenghe, Yanagida, Sako, Myadagsuren, Dorjsuren, Nakaya, Nakao, Ishikawa, Davaajav and Dulmaa2010; Li et al. Reference Li, Qiu, Yang, Craig, Chen, Xiao, Ito, Giraudoux, Wulamu, Yu and Schantz2005; Yang et al. Reference Yang, Sun, Li, Zhang, Teng, Liu, Liu, Zhao, Jones, Wang, Wen, Feng, Zhao, Zhao, Shi, Bartholomot, Vuitton, Pleydell, Giraudoux, Ito, Danson, Boufana, Craig, Williams and McManus2006; Torgerson et al. Reference Torgerson, Rosenheim, Tanner, Ziadinov, Grimm, Brunner, Shaiken, Shaikenov, Rysmukhambetova and Deplazes2009; Ziadinov et al. Reference Ziadinov, Deplazes, Mathis, Mutunova, Abdykerimov, Nurgaziev and Torgerson2011; Konyaev et al. Reference Konyaev, Yanagida, Ingovatova, Shoikhet, Nakao, Sako, Bondarev and Ito2012). Although the distribution range of the parasite is described as being widespread in the northern hemisphere, large regional gaps and differences are observed. For instance, in Europe, the epidemiological status of E. multilocularis has changed since the 1990s with a large continental extension of its range. However, it still remains patchy with large discontinuities of occurrence within and between the traditional high endemicity areas of mid-altitude mountains of the Alpine arc, its surroundings and newly discovered foci of northern Europe (Combes et al. Reference Combes, Comte, Raton, Raoul, Boué, Umhang, Favier, Dunoyer, Woronoff and Giraudoux2012; Davidson et al. Reference Davidson, Romig, Jenkins, Tryland and Robertson2012). Human AE cases in France are over-dispersed in a nested hierarchy of clusters varying in both space and time (Said Ali et al. – see this Special Issue of Parasitology, Vol. 140, 2013). Within a hotspot of higher endemicity, fine grain spatial distribution of the parasite and its hosts are still clustered. For instance, in a study carried out in eastern France, Raoul et al. (Reference Raoul, Deplazes, Nonaka, Piarroux, Vuitton and Giraudoux2001) found that 12% of fox definitive hosts harbour 76% of the adult parasite biomass. Furthermore, over the same extensive area, although the average prevalence in vole intermediate hosts was usually <0·01%, it could be >100x larger in some local foci (Giraudoux et al. Reference Giraudoux, Delattre, Takahashi, Raoul, Quere, Craig, Vuitton, Craig and Pawlowski2002). Similar patterns were also observed in Switzerland (Hofer et al. Reference Hofer, Gloor, Müller, Mathis, Hegglin and Deplazes2000; Burlet et al. Reference Burlet, Deplazes and Hegglin2011) and Kyrgyzstan (Ziadinov et al. Reference Ziadinov, Deplazes, Mathis, Mutunova, Abdykerimov, Nurgaziev and Torgerson2011).
The patchy distribution of E. multilocularis may reflect a variable distribution of favourable environments conducive to more intensive transmission. The distribution range of possible definitive hosts covers virtually the totality of the northern hemisphere, and susceptible intermediate host species (>40 to date) are virtually present in every possible terrestrial habitat of that area (Giraudoux et al. Reference Giraudoux, Delattre, Takahashi, Raoul, Quere, Craig, Vuitton, Craig and Pawlowski2002; Vuitton et al. Reference Vuitton, Zhou, Bresson-Hadni, Wang, Piarroux, Raoul and Giraudoux2003). Temperature and relative humidity have an impact on tapeworm egg survival (Veit et al. Reference Veit, Bilger, Schad, Schäfer, Frank and Lucius1995) and may be important limiting factors at various scales (Burlet et al. Reference Burlet, Deplazes and Hegglin2011). Moreover, correlations between land cover and spatial distribution of human AE have been evidenced in Eastern France, in South Gansu, in South Ningxia and on the Tibetan plateau of western Sichuan and Qinghai (Giraudoux et al. Reference Giraudoux, Craig, Delattre, Bartholomot, Bao, Barnish, Harraga, Quéré, Raoul, Wang, Shi and Vuitton2003, Reference Giraudoux, Pleydell, Raoul, Quere, Wang, Yang, Vuitton, Qiu, Yang and Craig2006, Reference Giraudoux, Raoul, Pleydell, Li, Han, Qiu, Xie, Wang, Ito and Craig2013; Wang et al. Reference Wang, Vuitton, Qiu, Giraudoux, Xiao, Schantz, Raoul, Li, Yang and Craig2004, Reference Wang, Vuitton, Xiao, Budke, Campos-Ponce, Schantz, Raoul, Yang, Craig and Giraudoux2006; Pleydell et al. Reference Pleydell, Yang, Wang, Raoul, Danson, Craig, Vuitton and Giraudoux2008). A similar correlation has been found for fox infection in France (Pleydell et al. Reference Pleydell, Raoul, Danson, Graham, Craig, Tourneux and Giraudoux2004). Those correlations have been explained by the impact of land cover on intermediate host population dynamics. The amplitude of seasonal and inter-annual variations of population densities of some arvicolid and/or lagomorph small mammal species increases with the ratio of their optimal habitat to the total available habitat (Lidicker, Reference Lidicker2000). This may lead to phases of large population densities where the biomass of the total population of intermediate host can reach several tens of kilograms per ha. During those periods, fox definitive hosts feed almost exclusively on those prey populations that are easy to access and are more likely to ingest infected small mammals even when prevalence is low (Raoul et al. Reference Raoul, Deplazes, Rieffel, Lambert and Giraudoux2010). These factors foster transmission (Giraudoux et al. Reference Giraudoux, Delattre, Takahashi, Raoul, Quere, Craig, Vuitton, Craig and Pawlowski2002) and human exposure risk (Viel et al. Reference Viel, Giraudoux, Abrial and Bresson-Hadni1999), the latter possibly via dogs feeding on abundant reservoirs of small mammal intermediate hosts close to towns, villages and rural settlements. However, even in areas where an overall higher prevalence of human AE was observed and the combination of apparent favourable climate and landscape was met, there still remained large unexplained differences between sampling units (generally villages) (Danson et al. Reference Danson, Graham, Pleydell, Campos-Ponce, Giraudoux and Craig2003, Reference Danson, Craig, Man, Shi and Giraudoux2004).
If landscape metrics could be used to predict areas of higher risk for E. multilocularis transmission, a key factor will be the presence of small mammal communities that are likely to include intermediate host species prone to reach large population densities within suitable habitats, in addition to climate variables that are likely to favour parasite egg survival (Giraudoux et al. Reference Giraudoux, Raoul, Pleydell, Li, Han, Qiu, Xie, Wang, Ito and Craig2013). Furthermore, within those areas predicted at higher risk, large unexplained differences between localities must be expected, due to the typical over-dispersed pattern of this helminth parasite. This can lead to discrete areas of 100–1000 square kilometres where human AE prevalence is more than 10–100 times larger than outside the high-risk zone. Those areas can be considered ‘hotspots’.
One particular feature of China and neighbouring countries of Central Asia, in contrast to Europe, is the extraordinary large variety of small mammal species and assemblages that covers this large region. Based on the comparison of mammal and plant species, a total of 25 biogeographical regions and 77 sub-regions have been defined in China (Xie et al. Reference Xie, Mackinnon and Li2004), including more than 274 species of small mammals many of which are candidate intermediate hosts for E. multilocularis transmission. However, it has been shown that E. multilocularis transmission, although sustained in a large number of different small mammal communities, does not depend on intermediate host species richness, but rather on relatively low biodiverse communities in which some species are prone to significant population increase (Giraudoux et al. Reference Giraudoux, Raoul, Pleydell, Li, Han, Qiu, Xie, Wang, Ito and Craig2013).
In this article, we use our field experience and knowledge (over the past 20 years) about the ecology of different E. multilocularis/human AE transmission hotspots to attempt to define ecological conditions that characterize those optimal transmission ecosystems in China and neighbouring countries of Central Asia.
HUMAN AE HOTSPOTS IN CHINA AND NEIGHBOURING COUNTRIES
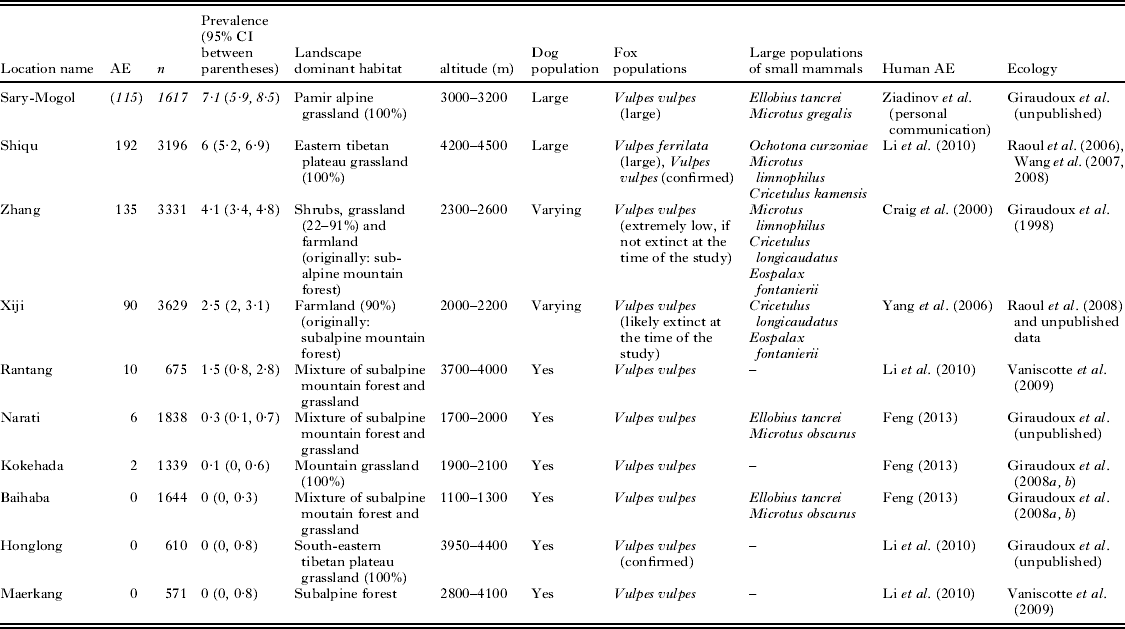
In parallel to host ecology studies, mass screenings for human AE were carried out in known AE endemic sites (Fig. 1). Table 1 summarizes the main features of the transmission systems prevailing in each study site and three of them, Sary-Mogol (Kyrgyzstan), Shiqu (China) and Zhang (China) showed much greater human AE prevalence than the others. Five sites in western China (Narati, Hoboksar, Baihaba, Honglong and Maerkang) showed human AE prevalence about 10 times lower, while Xiji and Rangtang sites in China could be ranked intermediate. Clearly Sary-Mogol, Shiqu, Zhang and also Xiji were transmission hotspots, with human AE prevalence exceeding by as much as 10 times the average prevalence reported for the region as a whole. The whole range of those sites is actually included in the range of the main definitive host of E. multilocularis, the red fox, Vulpes vulpes. However, on the Tibetan plateau the parasite is likely to circulate mostly through the Tibetan sand fox, V. ferrilata (Jiang et al. Reference Jiang, Liu, Zhang, Renqing, Xie, Li, Wang and Wang2012). Furthermore, the corsac fox, V. corsac, may be present in the drier areas of north China, and has also been found infected in Inner Mongolia and Kazahkstan (Xu et al. Reference Xu, Liance, Lin, Li and Houin1992; Bessonov, Reference Bessonov1998; Shaikenov and Torgerson, Reference Shaikenov, Torgerson, Craig and Pawlowski2002; Tang et al. Reference Tang, Quian, Kang, Cui, Lu, Shu, Wang and Tang2004, Reference Tang, Wang, Peng, Tang and Chen2006).

Fig. 1. Map of China and main locations cited in the text. The background is the Global Land One-kilometre Base Elevation model, provided by the US National Oceanic and Atmospheric Administration. Coordinates reference system in degrees (WGS84).KGZ, Kyrgyzstan; NX, Ningxia; Rt, Rangtang.
Table 1. Main characteristics of the areas studied. AE, number of human alveolar echinococcosis cases; n, number of people examined; in Sary-Mogol area (ae cases between parentheses and numbers in italics), only unpublished first estimations from ultrasound mass screenings carried out in 2012 by one of the co-authors (IZ) and co-workers are available: further investigations and PCR confirmations are yet currently in process

All these transmission hotspots share the fact that a number of small mammal intermediate host species were prone to population outbreaks during the study period, indicated by large trapping success and small mammal activity indices (Giraudoux et al. Reference Giraudoux, Quere, Delattre, Bao, Wang, Shi, Vuitton and Craig1998; Raoul et al. Reference Raoul, Quere, Rieffel, Bernard, Takahashi, Scheifler, Wang, Qiu, Yang, Craig, Ito and Giraudoux2006, Reference Raoul, Pleydell, Quere, Vaniscotte, Rieffel, Takahashi, Bernard, Wang, Dobigny, Galbreath and Giraudoux2008). Small mammal population surges were also found in Narati and Baihaba in Xinjiang (China), however they appeared not to translate into human AE prevalence levels as large as in the other sites highlighted above. Rangtang is situated less than some tens of kilometres from the high altitude grassland of the eastern Tibetan plateau where known population outbreaks occur for the plateau pika, Ochotona curzoniae and arvicolid species (e.g. Banma area, see Giraudoux et al. (Reference Giraudoux, Pleydell, Raoul, Quere, Wang, Yang, Vuitton, Qiu, Yang and Craig2006)). It is probable that the small mammal survey we conducted in this area was carried out during a transitory low density phase of small mammal population fluctuation (Vaniscotte et al. Reference Vaniscotte, Pleydell, Raoul, Quere, Qiu, Qian, Li, Bernard, Coeurdassier, Delattre, Takahashi, Weidmann and Giraudoux2009). However, Rangtang is in the vicinity of a larger scale region where small mammal population densities were higher, and the exposure of Tibetan nomads travelling on the plateau may explain the unexpectedly large human AE prevalence we observed there.
Clearly the human AE disease hotspots reported in this review represent transmission ecology characteristics favourable to population surges of some small mammal reservoirs (Fig. 2). In Shiqu (eastern Tibetan plateau, Sichuan, China) and Sary-Mogol (southern Kyrgyzstan) transmission landcapes were characterized by alpine grassland (Giraudoux et al. Reference Giraudoux, Raoul, Pleydell, Li, Han, Qiu, Xie, Wang, Ito and Craig2013 and unpublished), while in Zhang (south Gansu, China), transmission was occurring in a mixed landscape of sub-alpine shrub, grassland and farmland resulting from massive deforestation (with greater AE prevalence in villages with larger ratio of shrub and grassland) (Giraudoux et al. Reference Giraudoux, Craig, Delattre, Bartholomot, Bao, Barnish, Harraga, Quéré, Raoul, Wang, Shi and Vuitton2003). In Xiji (south Ningxia, China), the landscape was largely dominated by terraced farmland and grassland developed after clearance of sub-alpine forests whose degraded remnants could be found only on the top of local hills (Pleydell et al. Reference Pleydell, Yang, Wang, Raoul, Danson, Craig, Vuitton and Giraudoux2008; Yang et al. Reference Yang, Clements, Gray, Atkinson, Williams, Barnes and McManus2012). In all of those landscapes, the permanent (e.g. Tibetan plateau) or transitory (e.g. during deforestation processes in Zhang and Xiji) extension of habitats favourable to cyclic species of small mammals was a key factor for E. multilocularis transmission. This fits quite well with observations made in western Europe, where arvicolid population outbreaks and larger prevalence of E. multilocularis in red fox populations and of human AE, correlated with the extension of permanent grassland in mid-altitude mountains (Viel et al. Reference Viel, Giraudoux, Abrial and Bresson-Hadni1999; Giraudoux et al. Reference Giraudoux, Craig, Delattre, Bartholomot, Bao, Barnish, Harraga, Quéré, Raoul, Wang, Shi and Vuitton2003; Pleydell et al. Reference Pleydell, Raoul, Danson, Graham, Craig, Tourneux and Giraudoux2004). A continental-wide increase of red fox population density after rabies vaccination campaigns has further complicated this pattern (Chautan et al. Reference Chautan, Pontier and Artois2000; Schweiger et al. Reference Schweiger, Ammann, Candinas, Clavien, Eckert, Gottstein, Halkic, Muellhaupt, Prinz, Reichen, Tarr, Torgerson and Deplazes2007) increasing E. multilocularis prevalence in the known endemic areas and extending areas of large prevalence to new areas with no small mammal population outbreaks.

Fig. 2. View of some of the hotspots studied. a, Outbreak of Arvicola terrestris and Microtus arvalis in the Jura massif grassland, France (tumuli are earth expelled by A. terrestris when digging galleries), b, outbreak of Ellobius tancrei, Sary-Mogol grassland, south Kyrgyzstan (note the typical conical tumuli with lateral opening); c, typical habitat of Microtus gregalis along streams, Sary-Mogol, south Kyrgyzstan; d, outbreak of Ellobius tancrei and Microtus obscurus, Narati, Tien Shan, Xingjiang, China; e, outbreak of Ochotona curzoniae, Tuanji, eastern Tibetan plateau, Sichuan, China; f, field terraces, Xiji, Ningxia; g, grassland and afforested areas in Xiji. Field terraces and early stages of recently afforested areas are typical habitats for Eospalax fontanierii and Cricetulus longicaudatus; h, in 1996, field terraces of Ban Ban Wan (human AE prevalence: 16%) and deforestation in progress on the other side of the valley, Zhang, Gansu, China.
In China and some neighbouring central Asian countries, endemic landscapes are associated with different small mammal communities belonging to various biogeographic areas (Giraudoux et al. Reference Giraudoux, Pleydell, Raoul, Quere, Wang, Yang, Vuitton, Qiu, Yang and Craig2006, Reference Giraudoux, Raoul, Pleydell, Li, Han, Qiu, Xie, Wang, Ito and Craig2013). On this basis, we hypothesise that transmission hotspots of E. multilocularis may occur in favourable climatic areas where large areas of grasslands (e.g. alpine, sub-alpine or plain) can be found and that meet the distribution range of rodent and/or ochotonid species prone to population surges. We believe this assumption can serve to establish risk maps on a continental scale and guide further research on human AE distribution at finer spatial resolution. It may also help to define transmission ecosystems using some species of small mammal as flagship or indicator species that are species signalling ecological conditions by which transmission of E. multilocularis may be sustained more intensively.
TOWARDS PREDICTING AT RISK AREAS AT CONTINENTAL SCALES
Data and methods
Keystone, flagship and umbrella species are conservation buzzwords to serve as shortcuts or proxies for conserving background biodiversity (Barua, Reference Barua2011). In conservation, flagship species are ‘popular charismatic species that serve as symbols and rallying points to stimulate conservation awareness and action’ (Heywood, Reference Heywood1995). Here we propose to apply this concept to parasite ecology, as small mammal host species that can serve to signal ecosystems in which E. multilocularis transmission can be especially fostered, and promote them to public health awareness. We do not mean that a flagship species is necessarily the main route for transmission, although it can often be the case (it would therefore also be a keystone species for transmission). In our case, ideally, a flagship species should be easy to detect and to identify, linked as much as possible with a specific ecosystem that has a high transmission potential (due to the presence of this species or due to the fact that other intermediate host species with a high transmission potential are associated with this species). Furthermore, their distribution area should not or only marginally overlap on a regional scale with other flagship species. Here, species selected as flagship species were either those shown to be subject to population outbreaks in our studies (Table 1) or from the literature (Tang et al. Reference Tang, Quian, Kang, Cui, Lu, Shu, Wang and Tang2004; Winters et al. Reference Winters, Rumbeiha, Winterstein, Fine, Munkhtsog and Hickling2010), easier to identify and typical of the regional small mammal assemblages. Data on small mammal and carnivore species range were provided by the International Union for Conservation of Nature (IUCN): http://www.iucnredlist.org and were updated based on Giraudoux et al. (Reference Giraudoux, Zhou, Quere, Raoul, Delattre, Volobouev, Déforêt, Ito, Mamuti, Scheifler and Craig2008b) in China and our own field observations in Kyrgyzstan. Species nomenclature was standardized according to Wilson and Reeder (Reference Wilson and Reeder2005), except for Microtus obscurus, the Altai vole, that has been recently shown to be different from M. arvalis, the common vole (Tougard et al. Reference Tougard, Montuire, Volobouev, Markova, Contet and Quéré2013).
Very few studies (and none in Asia) provide quantitative information on climatic conditions that may limit E. multilocularis distribution. Miterpakova et al. (Reference Miterpakova, Dubinsky, Reiterova and Stanko2006), in Slovakia, have shown that E. multilocularis distribution in foxes was limited by moderate to higher annual air temperature, lower annual rainfall and soil drought. Parasite prevalence was clearly much larger in areas with average annual temperatures lower than 9–12 °C. No evidence of E. multilocularis transmission in France was found where the average annual temperature was higher than 12 °C (Giraudoux, unpublished, derived from the 0·5 degree gridded dataset of the FAO Global maps (Leemans and Cramer, Reference Leemans and Cramer1991), http://www.fao.org/sd/EIdirect/climate/EIsp0002.htm). Here, in the current study in China and neighbouring countries of Central Asia, we assumed, for lack of better proxy to climatic conditions, that E. multilocularis egg survival was unlikely if average annual temperature was higher than 12 °C.
Information on land cover in China and neighbouring countries was obtained from the Global Land Cover 2000 project http://bioval.jrc.ec.europa.eu/products/glc2000/products.php. It was based on Spot Vegetation data from the period 1 January to 31 December 2000, and consisted of 31 land-cover classes mapped at a pixel resolution of 1 km. Here category 7 corresponds to the relatively rich grassland of the alpine and sub-alpine vegetation belt described above (see also Giraudoux et al. (Reference Giraudoux, Raoul, Pleydell, Li, Han, Qiu, Xie, Wang, Ito and Craig2013)). To take account of the possible effects of large areas of grassland in the surroundings of a given locations, a neighbourhood analysis was carried out. For every pixel of the map, we computed the ratio of grassland to the total area within a radius of 31 km.
RESULTS
The hotspots described above were all located in areas with an actual or potential larger percentage of grassland (Giraudoux et al. Reference Giraudoux, Quere, Delattre, Bao, Wang, Shi, Vuitton and Craig1998, Reference Giraudoux, Craig, Delattre, Bartholomot, Bao, Barnish, Harraga, Quéré, Raoul, Wang, Shi and Vuitton2003, Reference Giraudoux, Raoul, Pleydell, Li, Han, Qiu, Xie, Wang, Ito and Craig2013; Raoul et al. Reference Raoul, Quere, Rieffel, Bernard, Takahashi, Scheifler, Wang, Qiu, Yang, Craig, Ito and Giraudoux2006; Vaniscotte et al. Reference Vaniscotte, Pleydell, Raoul, Quere, Qiu, Qian, Li, Bernard, Coeurdassier, Delattre, Takahashi, Weidmann and Giraudoux2009). Furthermore, four major communities of small mammals can be identified in those areas which characterize a transmission system (Fig. 3).

Fig. 3. Grassland land cover and distribution range of flagship species, after IUCN (2012), modified.
Eastern Tibetan plateau
Grassland small mammal communities are characterized by the presence of O. curzoniae (plateau pika), selected here as the flagship species, because being diurnal it is easily detected and easy to identify, directly or based on its indices (holes, faeces, etc.). Furthermore its distribution range does not overlap other transmission systems. Its geographical distribution is grossly the same as that of Phaiomys leucurus (Blyth's mountain vole, formerly Microtus leucurus). On the eastern border of the plateau, some other species such as Microtus limnophilus (lacustrine vole), Lasiopodomys fuscus (smokey vole, formerly Microtus fuscus), Neodon irene (Irene's moutain vole, formerly Microtus irene), Cricetulus kamensis (Tibetan dwarf hamster) and Ochotona cansus (Gansu pika), can also locally reach large population densities (Raoul et al. Reference Raoul, Quere, Rieffel, Bernard, Takahashi, Scheifler, Wang, Qiu, Yang, Craig, Ito and Giraudoux2006) and contribute to transmission. All species except O. cansus have been found naturally infected with E. multilocularis. L. fuscus is confusingly still cited maybe erroneously in parasitology literature. Its actual distribution range appears to be limited to south Qinghai, while most citations likely concern P. leucurus, a widespread species quite similar morphologically. In fact, the two species were pooled into a single species in the past (Qiu et al. Reference Qiu, Liu, Schantz, Ito, Carol and He1999; Raoul et al. Reference Raoul, Quere, Rieffel, Bernard, Takahashi, Scheifler, Wang, Qiu, Yang, Craig, Ito and Giraudoux2006). Definitive hosts in the wildlife cycle are typically the Tibetan sand fox (V. ferrilata) and the red fox (V. vulpes) and both proved naturally infected (Jiang et al. Reference Jiang, Liu, Zhang, Renqing, Xie, Li, Wang and Wang2012). Dogs are also commonly infected in those areas (Budke et al. Reference Budke, Campos-Ponce, Qian and Torgerson2005a, Reference Budke, Jiamin, Craig and Torgersonb; Vaniscotte et al. Reference Vaniscotte, Raoul, Poulle, Romig, Dinkel, Takahashi, Guislain, Moss, Tiaoying, Wang, Qiu, Craig and Giraudoux2011). Observations of dog preying on small mammals in and around human settlements are common and small mammal consumption evidenced from dietary analysis (Wang et al. Reference Wang, Raoul, Budke, Craig, Xiao, Vuitton, Campos-Ponce, Qiu, Pleydell and Giraudoux2010). However, the details of the parasite transmission routes in this area are still far from complete. For instance, we do not know the relative contribution of the various intermediate host species to the parasite life-cycle. Possible inter-annual small mammal population density variations in both space and time may provide a meta-stable transmission system involving fox as well as dog definitive hosts (Giraudoux et al. Reference Giraudoux, Pleydell, Raoul, Quere, Wang, Yang, Vuitton, Qiu, Yang and Craig2006). However, the respective contribution of dog and fox in the cycle is still unclear locally. One may suspect that in some areas, most of the parasite population biomass is in the dog population in communities (Budke et al. Reference Budke, Campos-Ponce, Qian and Torgerson2005a). On the Tibetan plateau or elsewhere for that matter, evidence of a dog population infected with no link to a sylvatic cycle (involving foxes) is lacking, which poses the question of transmission sustainability in an isolated population of dogs. However, a recent dog reinfection study in a Tibetan area of Sichuan suggests that dog populations are reinfected quickly and may contribute substantially to an active peri-domestic cycle (Moss et al. – see this Special Issue of Parasitology Vol. 140, 2013).
Altai, Tien Shan and Pamir
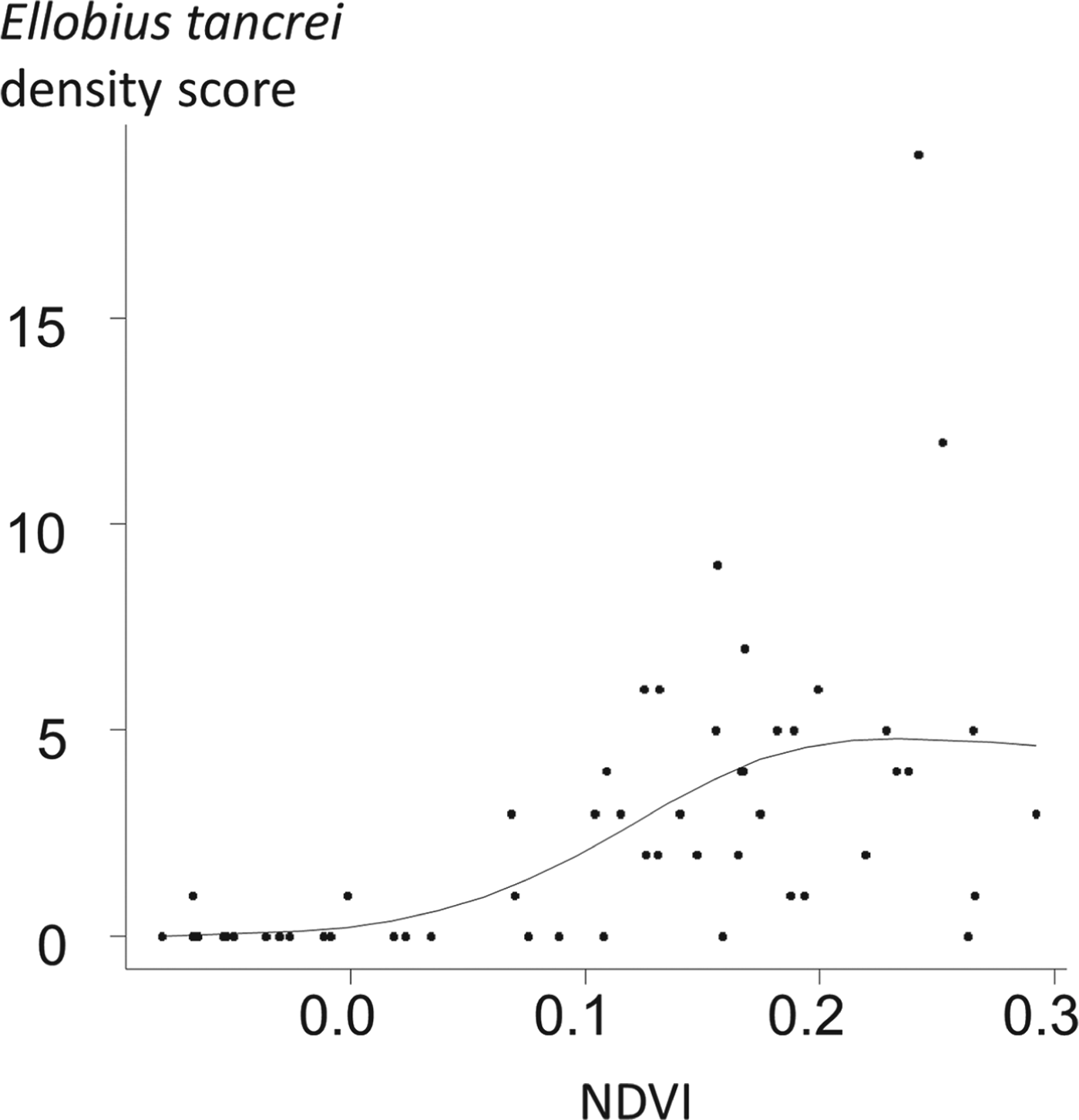
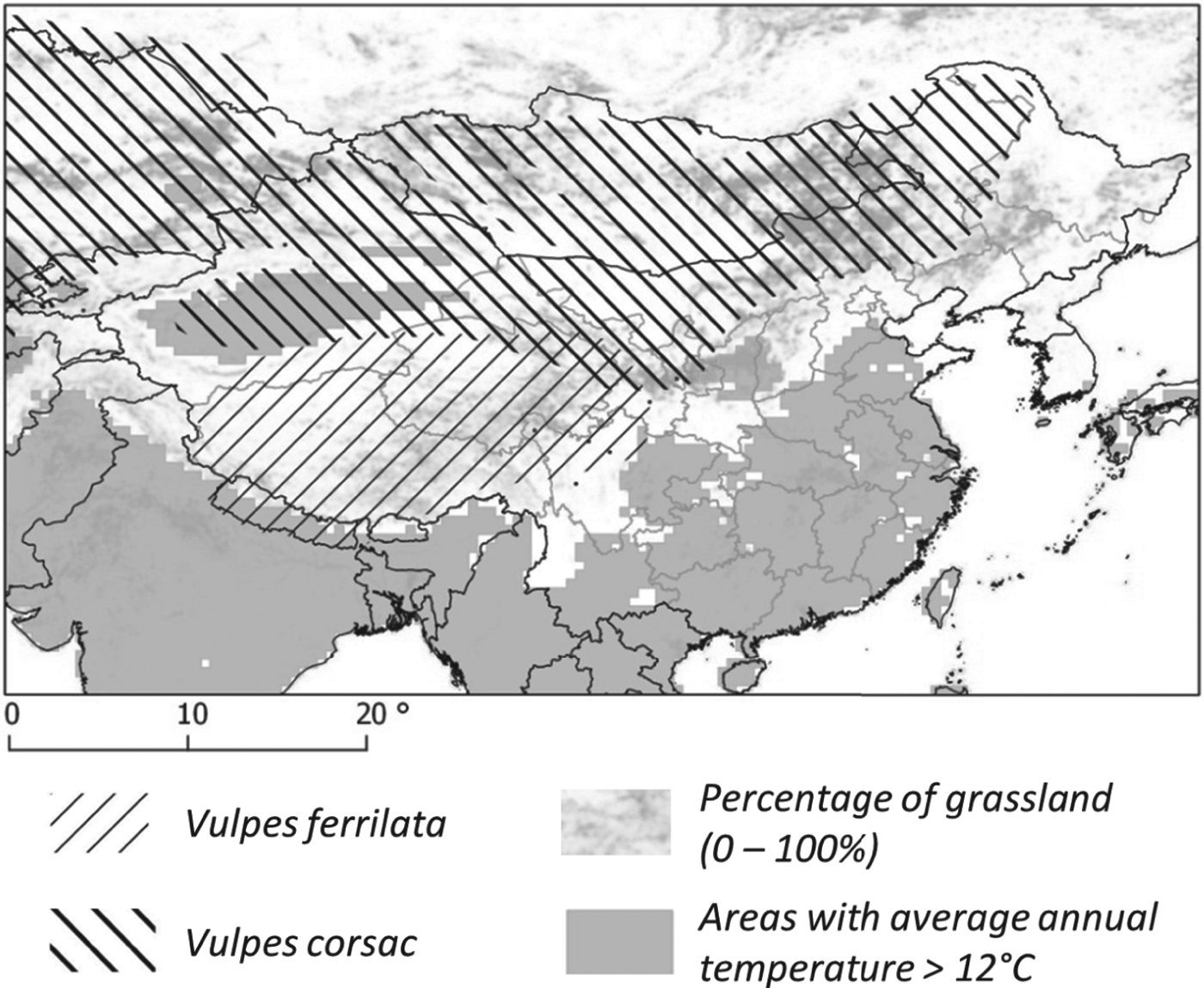
Ellobius tancrei (eastern mole vole) is a flagship species for small mammal communities and for an E. multilocularis transmission ecosystem, whose range stretches from the Pamir mountains (south Kyrgystan), through southern Kazakhstan to northern Xinjiang (Tien Shan and Altai mountains). It probably also includes western and central Mongolia where it meets the eastern small mammal assemblage (see below). This species is typical and easy to identify, directly or based on its cone-shaped earth tumuli with a middle hole from which a breach is maintained on one side. The population density of E. tancrei increases with grassland productivity (Fig. 4). It has been found naturally infected in Kyrgyzstan (Tokobaev, Reference Tokobaev1959; Afonso et al. unpublished). Additional potentially outbreaks of arvicolid species can be found more or less locally; for example, Microtus gregalis (narrow-headed vole), M. oeconomus (root vole) along streams and in marshes and M. obscurus (Altai vole), in grassland (Giraudoux et al. Reference Giraudoux, Zhou, Quere, Raoul, Delattre, Volobouev, Déforêt, Ito, Mamuti, Scheifler and Craig2008b; unpublished). The red fox is the typical definitive host in the wildlife cycle, and domestic dogs in villages, hamlets and isolated farms of the Alay valley (Kyrgyzstan) and in Mongol and Kazak settlements of northern Xinjiang. The contribution of the corsac fox (V. corsac) can be questioned locally (Fig. 5), but this species is generally not observed in mountainous areas or in regions where snow depth exceeds 150 mm. On this basis, it is not expected to be present in the alpine and sub-alpine grassland of the mountain ranges of this part of the world (Smith and Xie, Reference Smith and Xie2008; Wilson and Mittermeier, Reference Wilson and Mittermeier2009).

Fig. 4. Relative density of Ellobius tancrei population and grass density in Sary-Mogol, Alay Valley. 56 locations were selected at an average distance of 1·2 km from each other. 20 intervals of 10 paces were walked at each point in may 2012. The score of relative density of E. tancrei was the number of intervals where fresh E. tancrei indices could be observed (see e.g. Giraudoux et al. Reference Giraudoux, Pradier, Delattre, Deblay, Salvi and Defaut1995; Quéré et al. Reference Quéré, Raoul, Giraudoux and Delattre2000). The NDVI (Normalized Difference Vegetation Index), an index commonly used to estimate the relative density of plant cover, was computed from a Landsat ETM image acquired the 16th of June 2004. The relationship between E. tancrei score and NDVI was modelled using a general additive model with a Poisson error, and a thin plate regression smoother (using a three dimensional basis) (Wood, Reference Wood2006). The empirical variogram of residuals was included within the limits of the variogram envelop obtained from 99 random permutations of the original data, indicating that no spatial autocorrelation could be detected. The correlation between E. tancrei score and NDVI was found statistically significant at a probability lower than 0·00001.

Fig. 5. Distribution of fox species. The distribution of the red fox covers the totality of the area.
Eastern Mongolia and Inner Mongolia
Lasiopodomys brandtii (Brandt's vole, formerly Microtus brandtii) characterizes transmission systems of eastern Mongolia and eastern Inner Mongolia and has been found naturally infected (Tang et al. Reference Tang, Quian, Kang, Cui, Lu, Shu, Wang and Tang2004). It can reach large population densities and cause severe damage to grassland (Winters et al. Reference Winters, Rumbeiha, Winterstein, Fine, Munkhtsog and Hickling2010). The role and the importance of the Daurian pika (Ochotona dauurica), in this area are uncertain. This species is sometimes cited as a potential grassland pest in the literature (Komonen et al. Reference Komonen, Komonen and Otgonsuren2003) and has been found naturally infected in the Tuva Republic, Russia (Vuitton et al. Reference Vuitton, Zhou, Bresson-Hadni, Wang, Piarroux, Raoul and Giraudoux2003). Both the red fox and the corsac fox are present. Tang et al. (Reference Tang, Quian, Kang, Cui, Lu, Shu, Wang and Tang2004) provide evidence of E. multilocularis infection for the latter species, as well as for L. brandtii and Meriones unguiculatus (Mongolian jird).
South Gansu and south Ningxia
These provinces/autonomous regions of central-west China share very similar communities thriving in a mosaic of grassland and farmland originating from massive deforestation of sub-alpine forests until the early 1990s. In farmland and newly reforested areas they are dominated (in terms of biomass) by Cricetulus longicaudatus (long-tailed dwarf hamster) and Eospalax fontanierii (Chinese zokor) (Giraudoux et al. Reference Giraudoux, Quere, Delattre, Bao, Wang, Shi, Vuitton and Craig1998; Raoul et al. Reference Raoul, Pleydell, Quere, Vaniscotte, Rieffel, Takahashi, Bernard, Wang, Dobigny, Galbreath and Giraudoux2008), the latter species being selected here as flagship species. This species is easy to identify and well known by farmers who trap them actively in spring for crop protection. It leaves unmistakable indices such has holes of 10 cm diameter in fields and fallows, often closed by an earth cap. In the western part of the area (e.g. Gansu), M. limnophilus can also reach large population densities in shrubby and grassland areas. In southern Ningxia, we did not find evidence of arvicolid populations, except in the Liu Pan Mountains (Raoul et al. Reference Raoul, Pleydell, Quere, Vaniscotte, Rieffel, Takahashi, Bernard, Wang, Dobigny, Galbreath and Giraudoux2008; Giraudoux et al. personal observation in 2012). This suggests that south Ningxia may so far be the only place in the world, where E. multilocularis transmission (with resultant high human incidence) occurs without an arvicolid species being involved. The corsac fox is known to avoid cultivated land and although south Ningxia is included in the theoretical distribution range of V. corsac, one can assume the fox is absent in the human AE hotspots. Furthermore red fox populations, although present and still locally abundant in the 1980s, have considerably decreased and are virtually absent in areas of intensive farming such as in the human AE foci around Xiji (Yang et al. Reference Yang, Clements, Gray, Atkinson, Williams, Barnes and McManus2012, local farmer interviews, local CDC reports and Giraudoux et al. personal observation). Subsequently, the only possibility for the parasite to be maintained would be to circulate through a dog/zokor and/or a dog/hamster lifecycle (possibly involving locally a range of other species such as Spermophilus dauricus, the Daurian ground squirrel, and possibly some species of the Cricetinae, Gerbillinae, Dipodidae and Ochotonidae) (Raoul et al. Reference Raoul, Pleydell, Quere, Vaniscotte, Rieffel, Takahashi, Bernard, Wang, Dobigny, Galbreath and Giraudoux2008; Yang et al. Reference Yang, Clements, Gray, Atkinson, Williams, Barnes and McManus2012). As everywhere else in the world, dogs may themselves catch and eat small mammals. During a 2012 field survey in south Ningxia, ground squirrel populations had dramatically crashed and just one specimen could be observed whereas hundreds were visible in 2003 (Giraudoux et al. personal observation). In the early 1990s in Ningxia, people reportedly used to trap ground squirrels (Spermophilus spp.) for family meat consumption and questionnaires to human AE patients from south Ningxia indicated that viscera with multiple macro-cystic lesions on the liver were used to feed dogs (Li et al. Reference Li, Wang, Yin and Li1990; Wang et al. Reference Wang, Yin, Ma, Zhang, Zhang, Cheng, Jiang and Li1991). Furthermore, in spring, zokors (E. fontanierii) are usually caught in large numbers by farmers for crop protection and are also often given as food to dogs (local villagers and local CDC staff, personal communication). Moreover, Pleydell et al. (Reference Pleydell, Yang, Wang, Raoul, Danson, Craig, Vuitton and Giraudoux2008) found that drinking water not originating from tap or a well was a risk factor for human AE. This suggests that water possibly contaminated by dog faeces might be an additional source of human infection. Actually the general pattern for sustainable transmission of the parasite in south Ningxia is still more an enigma than in the other areas studied in China. South Ningxia may be the only place in the world yet described where E. multilocularis is maintained through a small mammal/dog cycle that is not connected to a sylvatic cycle involving foxes, but clear evidence of this is required.
Based on our knowledge gained in a number of E. multilocularis transmission hotspots studied in China and neighbouring countries of Central Asia, the aim of this review is to identify and describe the general characteristics of transmission ecosystems in that Region. The large complexity that may arise from the presence of hundreds of potential candidate small mammal (intermediate host) species can be overcome by consideration of a few flagship species of small mammal intermediate hosts that are prone to population surges and are embedded within suitable landscapes with an optimal combination of climate and land cover characteristics (Giraudoux et al. Reference Giraudoux, Raoul, Pleydell, Li, Han, Qiu, Xie, Wang, Ito and Craig2013). Using this approach, we have been able to identify 4 types of small mammal assemblages each characterized by a different flagship species; i.e. O. curzoniae, E. tancrei, L. brandtii and E. fontanierii (Fig. 6). These 4 small mammal species are all adapted to the cold climates typical of alpine and sub-alpine grasslands. In first three of them, small mammal population density is dependent on the response of grassland species to the availability and productivity of their grassland habitat within the given landscape. The fourth species, E. fontanierii, occurs in areas of south Ningxia (central China) which were originally mapped as sub-alpine forest or grassland. Those habitats have now been transformed into farmland. In such expanded agricultural areas, the only small mammal species that reaches large population biomass over large scales are zokors and hamsters, and these hosts probably indicate a unique transmission ecosystem within farmland where no arvicolid rodents appear to participate.

Fig. 6. Transmission systems and their flagship species. Except in the Eospalax fontanierii area (E.f.), more intense transmission and hotspots are more likely found in areas with larger ratio of permanent grassland. Question marks are placed on a number of areas of large ratio of permanent grassland but where no reference studies on small mammal communities and transmission are yet available.
Of course, this descriptive assessment is likely to be an oversimplification of reality. For instance, our knowledge of the details of the distribution range and ecology of small mammal host species is still very poor for much of central and eastern Eurasia (most IUCN maps available have been corrected based on data collected in our field surveys for this article). Furthermore, the concept of ‘flagship small mammal species’ and their association with a putative transmission system that we have proposed here must not hide the possible role of other key intermediate host species present in the target ecosystem and that could be involved in the sustainable transmission of E. multilocularis. In addition, undetected intermediate host species may occur whose detailed distributions will be poorly known or even unknown. For example, no description of an E. multilocularis transmission ecosystem has yet been proposed for the known areas of endemicity located in the southern part of Russia bordering China and Mongolia. In north-east China, for example, no single candidate flagship small mammal species can be proposed so far for the transmission system(s) responsible for local cases of human AE in Heilongjiang (Zhou, Reference Zhou2001; Danson et al. Reference Danson, Graham, Pleydell, Campos-Ponce, Giraudoux and Craig2003). There also remains much uncertainty about the general characteristics of transmission ecosystems where they overlap, e.g. between the Kunlun and Pamir mountains or in central Mongolia (Fig. 6).
Furthermore, large sciurid rodents such as Marmota spp., Spermophilus spp. and large lagomorphs, such as hares, are present in mountain grasslands and their populations locally may be relatively large. Because these mammals are relatively long lived intermediate hosts they may act as long-term reservoir hosts for E. multilocularis (Zhou et al. Reference Zhou, Wen, Wang, Delattre, Quere, Vuitton, Craig and Giraudoux1998, Reference Zhou, Chai, Craig, Delattre, Quere, Raoul, Vuitton, Wen and Giraudoux2000; Xiao et al. Reference Xiao, Li, Qiu, Nakao, Chen, Nakaya, Yamasaki, Schantz, Craig and Ito2004; Yang et al. Reference Yang, Clements, Gray, Atkinson, Williams, Barnes and McManus2012), compared to the rapid turnover of a vole population which is almost totally renewed each year. Another enigma is the possible role in sustainable transmission of this parasite in semi-desert small mammal species e.g. gerbils (Meriones spp.) jerboas (Allactaga spp. and Dipus spp.), hamsters, (Cricetulus spp.), and other candidate mammals. These small mammal communities of arid environments tend to stay at much lower population densities due to the low primary production of the ecosystem they inhabit and to periods of long seasonal drought (the latter unfavourable to parasite egg survival). However, these arid ecosystem rodent communities can possibly meet and partly overlap with other small mammal communities at the edge of their ecological distribution (e.g. along streams, see Shaikenov and Torgerson (Reference Shaikenov, Torgerson, Craig and Pawlowski2002) and Tang et al. (Reference Tang, Quian, Kang, Cui, Lu, Shu, Wang and Tang2004)). We do not know whether those arid-adapted small mammal populations can sustain the transmission of E. multilocularis by themselves in the long term or are only sporadically exposed from parasite spill-over, for example via fox movements, from neighbouring communities (e.g. arvicolids) or from ecosystems that are more favourable for intensive transmission.
CONCLUSIONS
A striking feature of the transmission ecology of E. multilocularis in China and in some neighbouring countries of Central Asia is the diversity of potential small mammal intermediate hosts and the range of ecological and anthropogenic drivers (e.g. climate, geography, grassland management, deforestation, reforestation) that directly or indirectly contribute to reinforce or limit the parasite lifecycle in sylvatic mammals. The distribution of small mammal assemblages, their ecological relationships, and even their basic taxonomy in China and Central Asia are far from completely understood, but the emergence of reasonably comprehensive atlases in term of species number and distribution have been published recently (Smith and Xie, Reference Smith and Xie2008; IUCN, 2012). Nevertheless, the lack of information about small mammal populations may be a considerable obstacle for understanding transmission patterns of E. multilocularis at relevant spatial scales, and thus for subsequent public health awareness and zoonosis prevention options.
Based on the knowledge acquired in areas of China and Central Asia, where both small mammal host ecology and the epidemiology of human AE were studied in parallel, we strongly recommend an ecological approach that defines large spatial units or transmission ecosystems of E. multilocularis wherein general and specific environmental features may indicate or even predict ‘risky’ or ‘unhealthy’ landscapes (Patz et al. Reference Patz, Daszak, Tabor, Aguirre, Pearl, Epstein, Wolfe, Kilpatrick, Foufopoulos, Molyneux and Bradley2004). Those units or ecoscapes (Lidicker, Reference Lidicker2008) can be characterized by flagship small mammal host species whose distribution corresponds to ecological conditions more likely to drive intensive transmission. This may happen when landscape characteristics and climatic conditions combine together with the extension of optimal habitats for some small mammal species prone to population outbreaks. The relative role of each of those particular small mammal species in transmission of E. multilocularis can hardly be quantified at this stage, not least because of the extreme complexity of their multi-annual population dynamics with a cascade of consequences on the host prey/predator relationships and on parasite transmission (Giraudoux et al. Reference Giraudoux, Pleydell, Raoul, Quere, Qian, Yang, Vuitton, Qiu, Yang and Craig2007; Raoul et al. Reference Raoul, Deplazes, Rieffel, Lambert and Giraudoux2010). Nevertheless, taken as whole, each putative transmission ecosystem with its own biotic and abiotic characteristics can serve as the basis for further in- depth research and in the long term help decision makers to implement surveillance systems and prevention for this pathogenic zoonosis.
ACKNOWLEDGMENTS
Thanks go to countless people who have regularly provided invaluable support and aid during our field expeditions in China and Kyrgyzstan.
FINANCIAL SUPPORT
Financial support from the Fogarty International Centre of the National Institutes of Health (Grants RO1 TW001565, R01 TW001665), from Grants-in-Aid for Scientific Research (nos. 14256001, 17256002, 21256003, 24256002) by the Japan Society for the Promotion of Science (JSPS), JSPS-Asia/Africa Science Platform Fund (2006–2008, 2009–2011), and Asian Science & Technology Strategic Cooperation Promotion Program by the Ministry of Education, Japan (2010–2012), from the Xinjiang Key Lab of Fundamental Medical Research on Echinococcosis of China (Grant no. XJDX0202-2004-01), from the National Natural Science Foundation of China (Grant 30960339), from the Australian National Health and Medical Research Council (Grant APP1009539) and from the Wellcome trust (Grant 15759). This research has been conducted within the context of the GDRI (International research network) ‘Ecosystem health and environmental disease ecology’. The content is solely the authors’ responsibility and does not necessarily represent the official views of the funders.