- BSG
-
brewers' spent grain
- COX-2
-
cyclooxygenase-isoform 2
- DPPH
-
1,1-diphenyl-2-picrylhydrazyl
Brewers' spent grain (BSG) is the solid fraction of barley malt remaining after the production of wort. According to the Eurostat Data, BSG is the main by-product of the brewing industry, with approximately 3·4 million tonnes being produced annually in the EU( Reference Stojceska, Ainsworth and Plunkett 1 ), at least 160 000 tonnes of which are produced in Ireland.
This solid residue contains water insoluble proteins in addition to the husk, pericarp and seed coat of the original barley grain( Reference Townsley 2 ). Protein and fibre account for 20 and 70% of BSG dry matter, respectively, while the starch content of BSG is insignificant (due to the absence of starchy endosperm). Owing to its protein-rich composition, BSG has the potential to be utilised in a manner similar to whey protein, providing health benefits for consumers. BSG is also rich in phenolic compounds, particularly ferulic acid and p-coumaric acid( Reference Bartolome, Santos and Jimenez 3 ), along with oligosaccharides and polysaccharides( Reference Mussatto, Dragone and Roberto 4 ). Emerging evidence, with regard to the ability of dietary phenolic compounds to exhibit anti-carcinogenic, anti-inflammatory and antioxidant activities( Reference Nagasaka, Chotimarkorn and Shafiqul 5 , Reference Yang, Landau and Huang 6 ) has led to significant interest in plant phenolic compounds particularly by the food industry, scientists and consumers.
To date, BSG has been widely used as an animal feed, particularly for cattle, to provide high amounts of both protein and fibre. BSG is an excellent feed ingredient for ruminants, providing all the essential amino acids when combined with inexpensive N sources such as urea( Reference Huige and Hardwick 7 ). However, with the increased cost of disposal of the solid fraction, alternative uses are highly sought-after and it has been shown that BSG can be effectively integrated into ready-to-eat snacks to increase dietary fibre, crude protein and fat levels( Reference Stojceska, Ainsworth and Plunkett 1 ). Other areas of successful research include the blending of BSG with flour for incorporation into cookies( Reference Prentice, Kissell and Lindsay 8 ) and the addition of BSG to dough to improve the dietary fibre content in bread( Reference Stojceska, Ainsworth and Plunkett 1 ).
This review details the existing evidence regarding BSG. A specific focus is placed on the potential bioactivities of phenolic compounds (particularly ferulic acid and p-coumaric acid) present in BSG, and the incorporation of BSG into foodstuffs, for both human and animal consumption.
Composition of brewers' spent grain
Many studies have reported on the approximate composition of BSG, which contains protein, fat, cellulose, hemicellulose and lignin (Table 1). As shown, there is good consistency with regard to the composition of BSG. However, variations can arise due to differences in barley variety, harvesting time, characteristics of hops added and brewery technology( Reference Santos, Jimenez and Bartolome 12 ). BSG predominantly consists of the husk-pericarp-seed coat layers that are rich in cellulose, non-cellulosic polysaccharides, lignin, protein and fat. This is reflected in the composition of BSG (Table 1), and thus BSG can be regarded as a lignocellulosic material( Reference Mussatto, Dragone and Roberto 4 ). In addition to the components detailed in Table 1, it has been shown that BSG is also a valuable source of vitamins, minerals and amino acids, particularly for animal feeding. The vitamins present in BSG are biotin, folic acid, niacin, choline, riboflavin and thiamine, pantothenic acid and pyroxidine( Reference Huige and Hardwick 7 ). BSG is also reported to contain minerals such as Ca, Cu, Fe, Mn, K and Na( Reference Huige and Hardwick 7 , Reference Pomeranz and Dikeman 17 ) and both essential (including lysine, histidine, methionine, phenylalanine, tryptophan) and non-essential (including alanine, serine, glycine, proline) amino acids( Reference Huige and Hardwick 7 ). When combined with inexpensive N sources, such as urea, BSG can provide all the essential amino acids to ruminant animals( Reference Huige and Hardwick 7 ).
Table 1. The approximate chemical composition of brewers' spent grain

* Values expressed as % dry matter, which has been documented in two studies to be 20·4( Reference Beldman, Hennekam and Voragen 9 ) and 20%( Reference El-Shafey, Gameiro and Correia 13 ).
Phenolic component of brewers' spent grain
Phenolics present in brewers' spent grain
Phenolic acids, particularly hydroxycinnamic acids and hydroxybenzoic acids are secondary plant metabolites found extensively in plant foods. Phenolic acids are currently the focus of much attention due to their potential to act as antioxidant, anti-inflammatory and anti-carcinogenic compounds( Reference Nagasaka, Chotimarkorn and Shafiqul 5 , Reference Yang, Landau and Huang 6 ).
As previously mentioned, BSG consists predominantly of the husk-pericarp-seed coat and is largely made up of cell walls. Since most of the phenolic compounds of the barley grain are contained in the husk( Reference Mussatto, Dragone and Roberto 4 ) and hydroxycinnamic acids accumulate in the cell walls, BSG is a potentially valuable source of phenolic acids.
There is evidence to suggest that ferulic acid and p-coumaric acid (as shown in Fig. 1) are present at relatively high concentrations in BSG( Reference Bartolome, Santos and Jimenez 3 ). Some of the existing literature regarding the presence of ferulic and p-coumaric acid is detailed in Table 2. Ferulic acid was found to be the most abundant hydroxycinnamic acid being present at concentrations ranging from 1860 to 1948 μg/g, while the p-coumaric levels ranged from 565 to 794 μg/g( Reference Hernanz, Nuñez and Sancho 23 ). More recent evidence shows that BSG consists of 1·16% mono and dimeric phenolic acids, with 53% of the monomeric phenolic acids accounted for by ferulic acid. The vast majority of phenolic acids were also found to be in the bound form( Reference Forssell, Kontkanen and Schols 20 ). It has been reported that following ferulic and p-coumaric acids, the next most abundant phenolic acids in BSG were found to be sinapic, caffeic and syringic acids( Reference Szwajgier, Wako and Targoski 22 ). A summary of the phenolic acids present in BSG is given in Table 3.

Fig. 1. The general structure of hydroxycinnamic acid and the functional groups for ferulic and p-coumaric acids.
Table 2. The percentage (% dry weight) of bound phenolics, ferulic acid and p-coumaric acid present in brewers' spent grain

Table 3. The most abundant phenolic acids present in brewers' spent grain modified from Szwajgier et al. ( Reference Szwajgier, Wako and Targoski 22 )
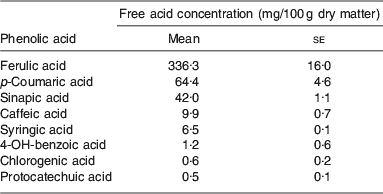
Extraction of phenolic acids from brewers' spent grain
Numerous studies have been conducted to extract phenolic acids from BSG. Novel techniques for extraction, such as a rapid microwave-assisted derivatisation process have been investigated( Reference Athanasios, Georgios and Michael 19 ). However, the majority of approaches use the basis of either acid hydrolysis or saponification (with 1–4 m NaOH) and liquid–liquid or liquid–solid extraction. A review of methods of extracting, separating and detecting phenolic acids in natural plant foods showed that the most frequently used methods involve acid hydrolysis and saponification( Reference Stalikas 24 ). Extraction usually entails the use of solvents such as methanol and ethyl acetate. TLC is extensively used for detecting phenolic acids due to its high sample throughput. However, using HPLC gives a greater degree of separation of compounds and is highly reproducible where quantification is possible. Therefore, reverse phase-HPLC is predominantly used, but HPLC coupled with UV or diode array detection is also an option( Reference Zgórka and Kawka 25 ). A review looking at the extraction and quantification of phenolics in foods also reported that methanolic extraction and alkaline hydrolysis are commonly used for phenolic acid extraction, while a sequential alkaline hydrolysis releases bound phenolics( Reference Naczk and Shahidi 26 ). A new method has recently been developed and validated, for the release of phenolic acids (both free and bound) from cereals including barley. This method uses solid-phase extraction coupled with HPLC-diode array detection analysis and is simple, inexpensive and gives good recoveries and precision( Reference Irakli, Samanidou and Biliaderis 27 ). Recently published results show that exogenous ferulic acid esterase produced by the probiotic organism Lactobacillus acidophilus K1 can successfully release the free phenolics from BSG( Reference Szwajgier 28 ). In 2005, a study looking at the hydroxycinnamate content of BSG fractions utilised saponification with 4 m NaOH. The supernatants were then neutralised and extracted with ethyl acetate, dried and re-suspended in MetOH:H2O( Reference Mandalari, Faulds and Sancho 18 ). More recent research also used this method, giving comparable results( Reference Robertson, I'Anson and Treimo 21 ). Using a LUNA C18 reverse phase-HPLC column, both studies found that ferulic acid was the phenolic acid in greatest abundance in BSG, with coumaric acid being second highest. Saponification (involving the treatment of samples with 1–4 m NaOH solution) has been widely used to extract hydroxycinnamic acids from BSG( Reference Bartolome, Santos and Jimenez 3 , Reference Hernanz, Nuñez and Sancho 23 , Reference Faulds, Mandalari and LoCurto 29 ).
Extraction methods similar to those used for BSG, have also been utilised with other materials such as wheat bran extracts( Reference Kim, Tsao and Yang 30 ) and apple waste extracts( Reference McCann, Gill and O'Brien 31 ).
Potential health benefits of phenolic component of brewers' spent grain
As previously mentioned, ferulic and p-coumaric acids are the phenolic acids at highest concentrations in BSG (Table 3). A lot of research has been conducted looking at the antioxidant activity of hydroxycinnamic acids, particularly ferulic and p-coumaric acids. A commonly used method for quantification of antioxidant activity is the 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay, which measures the ability of the test compound to scavenge the DPPH radical. Chen and Ho( Reference Chen and Ho 32 ) have shown the antioxidant potential of ferulic acid using the DPPH and Rancimat methods, but ferulic acid was a less potent antioxidant than caffeic acid and α-tocopherol( Reference Brand-Williams, Cuvelier and Berset 33 ). Caffeic acid has been shown to act as an antioxidant in vitro and scavenged radicals including DPPH and the superoxide anion( Reference Gulcin 34 ). It has also been shown, using the DPPH assay, that a number of hydroxycinnamic acids act as antioxidants, scavenging DPPH in the order caffeic acid > sinapic acid = ferulic acid > ferulates>p-coumaric acid( Reference Kikuzaki, Hisamoto and Hirose 35 ). Similarly, but using an alternative method, a study investigating the phenolic compounds in wheat bran extract and their antioxidant activity again found that ferulic acid was one of the strongest antioxidants using the β-carotene linoleic acid model system. The β-carotene linoleic acid model system assay is based on the principle that at a high temperature the oxidation of linoleic acid produces peroxides that decolourise β-carotene. The wheat bran extracts with highest ferulic acid concentrations (following alkaline hydrolysis) also exhibited higher antioxidant activity( Reference Kim, Tsao and Yang 30 ). Ferulic acid and caffeic acid have been reported to have excellent antioxidant potential at low concentrations, with the ability to scavenge a range of free radicals. Both phenolic acids scavenge the reactive oxygen species and reactive nitrogen species, with concentration-dependent scavenging of NO, superoxide and 2,20-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid radical. In line with the previously discussed evidence, caffeic acid was a stronger scavenger of the DPPH radical, but ferulic acid was better at scavenging 2,20-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid radical and NO( Reference Maurya and Devasagayam 36 ). In a recent study on beers, a direct correlation was found between ferric-reducing antioxidant power and a number of phenolic acids including ferulic, p-coumaric, caffeic, sinapic and vanillic acids( Reference Piazzon, Forte and Nardini 37 ). A second study also showed that some of the phenolic acids present in beer correlate with the antioxidant activity measured by the DPPH radical and superoxide anion scavenging, metal chelation and reducing power; these include syringic and caffeic acids( Reference Zhao, Chen and Lu 38 ). Since ferulic acid is so well recognised as an antioxidant, it is approved for use as a food additive in some countries to prevent oxidation( Reference Graf 39 , Reference Itagaki, Kurokawa and Nakata 40 ). In addition, it is important to note that while phenolic compounds can have an antioxidant effect, they have also been shown to act as pro-oxidants under certain conditions, thus inducing oxidative stress. Recent literature suggests that at low concentrations, many phenolics exhibit pro-oxidant behaviour, whereas the synthetic antioxidants, including α-tocopherol, do not( Reference Fukumoto and Mazza 41 ). For caffeic acid and ferulic acid to act as pro-oxidants, higher concentrations are required( Reference Maurya and Devasagayam 36 ). It has also been found by using the comet assay that at high concentrations p-coumaric acid enhanced DNA breakage induced by H2O2. This may be due to the production of reactive oxygen species by p-coumaric acid as a result of its pro-oxidant activity( Reference Ferguson, Zhu and Harris 42 ). It has been suggested that this pro-oxidant effect is related to the presence of metal ions in the body (for example due to tissue injury releasing Fe and Cu) and is of relevance for the bioactivity of phenolic compounds in vivo ( Reference Morton, Caccetta and Puddey 43 ). It is clear from a small number of in vivo studies that the hydroxycinnamic acids have antioxidant properties. Such studies are essential to understand the biological role of these phenolic acids( Reference Shahidi and Chandrasekara 44 ).
In addition to their antioxidant potential, there is increasing evidence to suggest that phenolic acids can have an anti-carcinogenic effect. Caffeic acid exhibits an anti-proliferative effect on several cancer cells including mammary gland, adenocarcinoma, lymphoblastic leukaemia( Reference Gomes, Girão da Cruz and Andrade 45 ) and cervical cancer cell lines as assessed using the MTT assay( Reference Gomes, Girão da Cruz and Andrade 45 , Reference Chang, Hsieh and Hsiao 46 ). The cyclooxygenase-isoform 2 (COX-2) assay has been used for determination of the anti-cancer potential of these compounds. Overexpression of COX-2 increases the conversion of arachidonic acid to prostaglandins, which are important mediators of inflammation, and are associated with cancer. Phenolic acids including caffeic acid( Reference Kang, Lee and Shin 47 ) and vanillic acid( Reference Kim, Kim and Kim 48 ), and polyphenols including epigallocatechin-3-gallate( Reference Hussain, Gupta and Adhami 49 ) and quercetin( Reference García-Mediavilla, Crespo and Collado 50 ), have been shown to inhibit the expression of COX-2, possibly reducing cancer risk. Apoptosis in cancer cell lines is also an indicator of anti-carcinogenic potential and can be assessed by a number of methods including DNA fragmentation and the Hoechst staining assay. Cinnamic acid derivatives induced apoptosis in human leukaemia (HL60) and colon cancer (SW480) cell lines, as measured by the aforementioned apoptosis methods( Reference Akao, Maruyama and Matsumoto 51 ). In addition, the anti-apoptotic effect of phenolic compounds including ferulic acid and caffeic acid on human peripheral blood mononuclear cells was investigated( Reference Khanduja, Avti and Kumar 52 ). Caffeic acid inhibited externalisation of phosphatidyl serine, which indicates the pre-apoptotic stages, and hence it was concluded to have an anti-apoptotic effect. DNA fragmentation was analysed using an apoptotic DNA ladder kit. It was shown that pre-treating cells with caffeic acid, ferulic acid or ellagic acid before exposure to H2O2 inhibited DNA fragmentation. Recently published data add to the evidence available in the area, by measuring the ability of phenolic compounds to modulate NF-κB activity. In the inflammatory process, NF-κB is a transcription factor, whose increased activation has been reported in several human cancers( Reference Escárcega, Fuentes-Alexandro and García-Carrasco 53 ). Free phenolic acids that can be found in cereal grains (including ferulic, caffeic, sinapic and p-coumaric acids) significantly modulate NF-κB activity in U9373xκB-LUC cells, with a desired level of modulation being achieved by the synergistic action of phenolic acids and other phenolic compounds( Reference Hole, Grimmer and Jensen 54 ). Animal studies have also been carried out to determine the anti-carcinogenic potential of phenolic acids. An animal study to establish the effect of curcumin, chlorogenic acid, ferulic acid and caffeic acid on tumour promotion in the skin of mice showed that chlorogenic, ferulic and caffeic acid prevented the number of 12-O-tetradecanoylphorbol-13-acetate-induced tumours per mouse by 60, 28 and 35%, respectively( Reference Huang, Smart and Wong 55 ). Results of a later animal study suggest that ferulic acid not only inhibits the growth of aberrant crypt foci in the colon but also prevents the conversion of pre-neoplasia to malignant neoplasia( Reference Kawabata, Yamamoto and Hara 56 ). A recently published review on plant phenolics reported that natural phenolics, including tea and fruit polyphenols, play an antagonistic role in all stages of cancer development and that further study on these compounds will provide information regarding their possible future pharmaceutical use( Reference Dai and Mumper 57 ).
Cytokines are small cell signalling molecules involved in the inflammatory response, these include interleukins and interferons (for example interferon-γ). The ability of a compound to alter the production of a stimulated cytokine or NO indicates the compound's potential to act as an immune-modulator. Murakami et al. ( Reference Murakami, Nakamura and Koshimizu 58 ) investigated the effect of both ferulic acid (from rice bran) and FA15 (a derivative of ferulic acid) on NO synthase, COX-2 and TNFα in the RAW264·7 murine macrophage cell line. Unlike the ferulic acid isolated from rice bran, the synthesised FA15 derivative was found to inhibit the release of TNFα and reduce the protein expression of both nitric oxide synthase and COX-2( Reference Murakami, Nakamura and Koshimizu 58 ). Ferulic acid has also been shown to inhibit macrophage inflammatory protein-2 and TNFα production, induced by lipopolysaccharide in a macrophage cell line. The effect, although dose-dependent, was very weak compared with the effect of dexamethasone (a well-known inhibitor of interleukins)( Reference Sakai, Ochiai and Nakajima 59 ). In Japanese Oriental medicines, Cimicifuga heracleifolia is often used as an anti-inflammatory drug. Ferulic acid has been shown to be among the main phenolic acids in C. heracleifolia ( Reference He, Pauli and Zheng 60 ). Sakai et al. ( Reference Sakai, Kawamata and Kogure 61 ) showed that ferulic acid and isoferulic acid could reduce macrophage inflammatory protein-2 production in a dose-dependent manner in RAW264·7 cells. It was suggested that ferulic acid and isoferulic acid are responsible, at least in part, for the anti-inflammatory properties of the C. heracleifolia drug( Reference Sakai, Kawamata and Kogure 61 ). Recently published data have shown that ferulic acid and p-coumaric acid inhibited lipopolysaccharide-induced NO production and inducible NO synthase in macrophages( Reference Kim, Min and Kwon 62 ). This supports earlier evidence suggesting that these phenolic acids can act as anti-inflammatory agents, by reducing TNFα induced IL-6 production in adipocytes. Quercetin, p-coumaric acid and reservatrol showed greatest inhibition of IL-6 production( Reference Yen, Chen and Chang 63 ).
Oxidised LDL is a well-recognised risk marker of CVD which is principally caused by atherosclerosis( Reference Yoshida and Kisugi 64 ). Evidence exists for the effect of hydroxycinnamic acids on the inhibition of LDL oxidation. Nardini et al. ( Reference Nardini, D'Aquino and Tomassi 65 ) demonstrated the antioxidant effect of hydroxycinnamic acid derivatives such as caffeic, ferulic and p-coumaric acids on LDL oxidation in vitro, with the use of the copper ion Cu2+ as a catalyst. At a concentration of 100 μm, all phenolic acids except p-coumaric acid inhibited LDL oxidation; at 20 μm, ferulic acid inhibited about 92% of Cu-catalysed human LDL oxidation; at 5 μm only caffeic acid strongly inhibited the oxidation of LDL( Reference Nardini, D'Aquino and Tomassi 65 ). A second study using similar methodology also found that both ferulic and p-coumaric acid showed a dose-dependent inhibition of human LDL oxidation in vitro when tested at 5, 10 and 20 μm ( Reference Meyer, Donovan and Pearson 66 ). In agreement with these data, results of a later study showed sinapic, ferulic and p-coumaric acids inhibited LDL oxidation( Reference Andreasen, Landbo and Christensen 67 ). Other compounds that have shown the ability to reduce Cu-induced LDL oxidation include catechin( Reference Chen, Lin and Liu 68 ).
Our research group is the first, to our knowledge, to look specifically at phenolic extracts from BSG. Four extracts from pale BSG (P1, P2, P3 and P4) and four extracts from black BSG (where the grain is roasted to 200 °C before brewing; B1, B2, B3 and B4) were analysed. Each extract results from a different step in the extraction process and hence contains different phenolic acid compositions. Extract 1 (P1, B1) contains free phenolics, extract 2 (P2, B2) contains bound phenolics, extract 3 (P3, B3) contains the remainder of bound phenolics and extract 4 (P4, B4) contains phenolics extracted with 110 mm NaOH( Reference McCarthy, O'Callaghan and Connolly 69 ). The ability of the phenolic extracts to protect against oxidant-induced DNA damage was determined using the comet assay. In the U937 cell line, oxidative DNA damage was induced by a range of oxidants; H2O2, 3-morpholinosydnonimine hydrochloride, 4-nitroquinoline oxide and tert-butylhydroperoxide. Table 4 shows the ability of the extracts to protect the cells against DNA damage. There was no protection against DNA damage induced by either 4-nitroquinoline oxide or tert-butylhydroperoxide. Ferulic acid and the black BSG extracts significantly reduced the DNA damage induced by H2O2, while P2, B2, B3 and B4 significantly reduced the percent tail DNA induced by 3-morpholinosydnonimine hydrochloride( Reference McCarthy, O'Callaghan and Connolly 69 ). The four oxidants used have different mechanisms of action; damage induced by both H2O2 and 3-morpholinosydnonimine hydrochloride involve the Fenton reaction which is an Fe-dependent reaction, 4-nitroquinoline oxide mimics the action of UV and Cu2+ plays an essential role, whereas Fe does not( Reference Yamamoto, Inoue and Kawanishi 70 ), tert-butylhydroperoxide causes lipid peroxidation and acts in a Ca2+-dependent manner and Fe plays less of a role than in H2O2-induced damage( Reference Kruszewski, Iwaneńko and Bartłomiejczyk 71 ). Therefore, BSG phenolic extracts may provide protection against oxidant-induced DNA damage by Fe chelation( Reference McCarthy, O'Callaghan and Connolly 69 ).
Table 4. DNA damage in U937 cells following 24 h incubation with 0·5% (v/v) pale (P1–P4) or black (B1–B4) brewers' spent grain phenolic extracts or 0·1 μg/ml ferulic acid (adapted from McCarthy et al. ( Reference McCarthy, O'Callaghan and Connolly 69 ))

SIN-1, 3-morpholinosydnonimine hydrochloride; 4-NQO, 4-nitroquinoline oxide; t-BOOH, tert-butylhydroperoxide.
* Data represent the mean of at least three independent experiments.
† Denotes a significant difference in DNA damage (P < 0·05), relative to oxidant control. Statistical analysis by ANOVA followed by Dunnett's test.
In summary, there is increasing evidence to suggest that phenolic acids, including those found at highest concentrations in BSG, can confer potential health benefits including anti-inflammatory, antioxidant, anti-carcinogenic and anti-atherogenic effects. Recent data suggest that BSG has antioxidant potential and therefore further research on the phenolic compounds extracted from BSG is warranted.
Incorporation of brewers' spent grain into feed/foodstuffs
Animal food
As previously mentioned, BSG contains approximately 20 and 70% protein and fibre, respectively, and it is due to this favourable chemical composition that it has great potential for use as a raw material/food ingredient( Reference Mussatto, Dragone and Roberto 4 ). BSG is an ingredient of significant importance for ruminants. When administered with low-cost N sources such as urea, BSG can supply all the essential amino acids to the ruminants. The effect of BSG on milk yield and composition and the blood components of dairy cattle has also been studied( Reference Belibasakis and Tsirgogianni 72 ). The cattle received a diet consisting of ground maize, maize silage, soya bean meal and wheat bran, with the latter three being substituted with wet brewer's grain in the treatment group. The study showed the treatment group had an increased milk yield, milkfat and milk total solids content. Blood components such as glucose, cholesterol, Na and TAG were not affected. While the main outlet for BSG is currently as a feedstuff for dairy cattle, research has also been conducted looking at the benefits of BSG for use as a feed for poultry and fish. The effect of replacing rice bran in a fish diet with 10–40% brewery waste grains has been investigated( Reference Kaur and Saxena 73 ). The brewery waste used contained 19% crude protein, 18–20% crude fibre and had a good amino acid profile. It was found that carp (oily freshwater fish) had better growth performance on diets containing brewery waste than the control group. The authors attributed this enhanced growth performance to the high-quality protein contained in the waste grains. A more recent study showed that biodegraded BSG contained cysteine, lysine and methionine in addition to fourteen other amino acids( Reference Essien and Udotong 74 ). Depending on the microbe used to degrade the BSG, different amino acid concentrations were found, with alanine consistently at highest concentrations. This composition was noted to be of particular importance for poultry as cysteine, lysine and methionine are the main amino acids required in poultry nutrition. For convenience, the possibility of producing dry BSG cakes suitable for long-term storage was examined using membrane filter press technology( Reference El-Shafey, Gameiro and Correia 13 ). These dry cakes could be used as an animal feed at any time, or as a starting material for the production of other products using BSG.
In summary, evidence suggests that whole BSG, fed as part of a total mixed ration, has many nutritional benefits for a range of animals, particularly dairy cattle. This has resulted in the routine use of BSG as an animal feed for cattle.
Human food
In addition to its use as an animal feed, BSG has been incorporated into foodstuffs for human consumption. Given its low cost and high nutritional value, BSG makes an ideal ingredient for human foods such as biscuits and ready-to-eat snacks, particularly where there is a need to increase fibre content. In 1978, the possibility of preparing cookies with flour containing BSG at levels ranging from 5 to 60% was examined( Reference Prentice, Kissell and Lindsay 8 ). At 40% BSG addition, the physical qualities of the cookies were sustained. This supplementation level gave a 74% increase in N and increased crude fibre ten-fold. These results were supported by work published in 2002, where the authors looked at the effect of BSG (at levels of 5–25%) on the fibre content and quality of cookies( Reference Öztürk 75 ). As the level of addition of BSG increased, there was a significant increase in dietary fibre content. Another documented foodstuff suitable for the inclusion of BSG is ready-to-eat snacks( Reference Stojceska, Ainsworth and Plunkett 1 ). BSG was added to the formulation mix (consisting of ingredients such as corn starch and wheat flour) at levels ranging from 10 to 30%. The incorporation was successful, increasing dietary fibre and crude protein levels. Similarly, the addition of BSG into extruded snack food has been studied( Reference Ainsworth, Ibanoglu and Plunkett 76 ). The maize flour of chickpea snacks was replaced with BSG at levels of 10, 20, 25 and 30%. The parameters measured included the effect of BSG supplementation on texture, colour, moisture, fat, fibre, starch, protein, phenolic compounds and antioxidant capacity. With increasing levels of BSG addition, the percent protein, fat and fibre content increased, while starch decreased. It was suggested that foods fortified with BSG be considered as functional foods. In a further study by Stojceska and Ainsworth( Reference Stojceska and Ainsworth 77 ), BSG was incorporated at different levels (0–30%) into wheat flour breads treated with four different enzymes and the bread quality was then evaluated( Reference Stojceska and Ainsworth 77 ). Similar to the previous study, it was found that the fibre content of the breads was significantly increased by BSG addition. The change in fat content was significantly linked to the addition of BSG. When addition of BSG is combined with the appropriate use of enzymes, the shelf life, texture and loaf volume can also be improved. Initially, it was thought that BSG was too granular for direct addition to food and that it would have to first be converted to flour before use. However, a study in 2009 demonstrated that BSG of various particle sizes could be effectively used in the production of frankfurters( Reference Özvural, Vural and Gökbulut 78 ). The control frankfurter had the highest score for acceptability, but the other products also had high scores, with the score decreasing with increase in particle size and reduction in fat levels. The authors suggested that BSG be used to produce low-fat high-fibre meat products. In addition to particle size, there are a number of points for consideration with the incorporation of BSG into foodstuffs. Firstly, there are concerns about appearance. When moist, BSG is brown in colour, thus it can only be effectively integrated into off-white products. Such foods include cookies and cakes. More importantly, it is imperative that the organoleptic properties of the foodstuff remain acceptable to consumers and are similar to the current commercially available products. The study by Prentice et al. ( Reference Prentice, Kissell and Lindsay 8 ), demonstrated that BSG addition at a level of 15% was the upper limit for organoleptic acceptability( Reference Prentice, Kissell and Lindsay 8 ). At this level, the organoleptic quality was lowered but still remained acceptable to consumer panels. Similarly, Stojceska et al. ( Reference Stojceska, Ainsworth and Plunkett 1 ) found that there was a limit to acceptability( Reference Stojceska, Ainsworth and Plunkett 1 ). At a level of addition of 30%, physicochemical characteristics (such as texture, colour and hardness) remained acceptable. However, the authors concluded that addition of BSG at 20% level was optimal to maintain properties of commercially available snack foods. Where the protein hydrolysates are to be extracted and incorporated into foodstuffs, there is concern over the bitter taste of some peptides, due to the hydrophobic amino acid content( Reference Clemente 79 ).
Conclusion
The literature shows that phenolic compounds including ferulic, caffeic and p-coumaric acid can have antioxidant, anti-cancer, anti-atherogenic and anti-inflammatory effects. Given that these phenolic acids are some of the major phenolics in BSG, it is expected that phenolic extracts from spent grain may also exhibit similar properties and have the potential to be developed for a range of bioactivities. BSG currently functions as an animal feed, having many nutritional benefits. While some attempts have been made to incorporate the bioactive components of BSG into foodstuffs, further research in this area is needed. Given the potential bioactive nature of the phenolic extracts from BSG, and the large amounts of BSG produced annually as a low value co-product, it is imperative that an alternative use be explored.
Acknowledgements
Funding for this research was provided under the National Development Plan, through the Food Institutional Research Measure, administered by the Department of Agriculture, Food and the Marine, Ireland. The authors declare no conflict of interest. A. L. M. wrote the manuscript with contributions from Y. C. O'C., C. O. P., R. J. F. and N. M. O'B.







